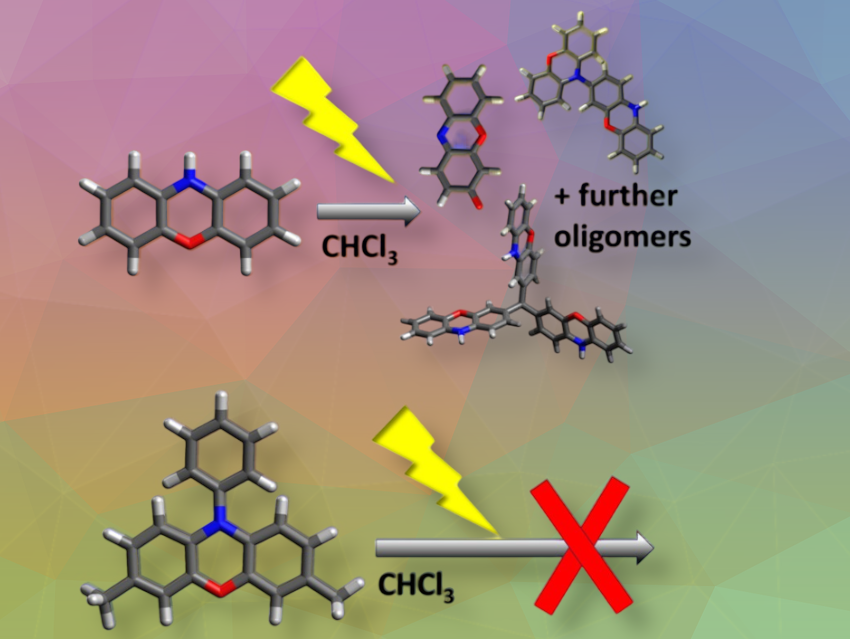
Phenoxazine is a common molecular building block used, for example, in optoelectronics and pharmaceuticals. However, it degrades quickly when exposed to light, especially in halogenated solvents. Ulrich Ziener and colleagues at Ulm University, Germany, studied the degradation products in both halogenated and non-halogenated solvents.
What did you do?
We investigated how phenoxazine, an electron-rich heteroaromatic molecule often used in pharmaceuticals or optoelectronic materials, for example, behaves in solution when treated with light. We focused on halogenated solvents, in particular chloroform, and irradiated the solutions with UV light, which led to a strong discoloration due to the degradation of phenoxazine.
Using spectroscopic methods, we were able to partially elucidate the degradation products and determine the relative stability of phenoxazine and differently substituted derivatives.
What got you interested in this?
When using this compound as a common donor moiety in donor-acceptor molecules, we observed a strong discoloration of the solutions within seconds, which also caused a clear emission. This is a highly undesirable property of such compounds for various applications such as organic light-emitting diodes (OLEDs). Although it is known that phenoxazine is photosensitive, neither the degradation mechanism nor measures against it are known. This was the starting point of the investigations.
What is new about it?
For the first time, we were able to show that defined oligomers of phenoxazine, which contain an additional carbon atom that probably originates from the solvent molecules, are formed upon irradiation of a chloroform solution.
In addition, it was thought in the community that the photostability of phenoxazine could be significantly improved by substitution at the nitrogen atom, e.g., by aromatic compounds. In contrast, we found that the N-phenyl-substituted derivative is less stable than the parent molecule.
What is the main significance of these results?
The study helps to understand how phenoxazine is degraded in halogenated solvents under the influence of light by a radical mechanism and how this degradation can be largely suppressed.
The results should make researchers working with phenoxazine and derivatives more sensitive to the handling of these compounds during synthesis and processing.
The study should also help to improve the stability of phenoxazine derivatives in optoelectronic applications such as OLEDs to increase the lifetime of such devices.
What part of your work was the most challenging?
The most difficult part of the investigations was to identify the various degradation products and to bring together seemingly contradictory findings to form a coherent picture, i.e., a convincing mechanism of photodegradation. As the degradation processes are poorly controlled, it is like looking for a needle in a haystack without knowing exactly what the needle looks like.
Thank you for these insights.
The article they talked about
- Photostability of Phenoxazine Derivatives,
Felix D. Goll, Philipp Welscher, Franz Bodenmüller, Remi Blinder, Fedor Jelezko, Ulrich Ziener,
ChemPhysChem 2024.
https://doi.org/10.1002/cphc.202400506

Professor Ulrich Ziener is Deputy Director of the Institute of Organic Chemistry III at Ulm University, Germany. (Photo: Elvira Eberhardt/Uni Ulm)