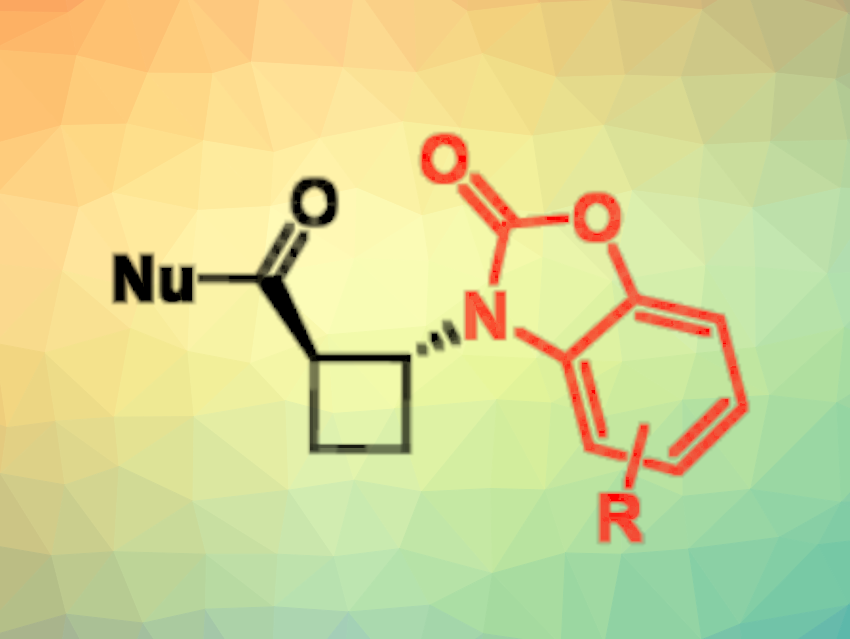
Cyclobutane β-amino acids are useful building blocks, e.g., for the construction of peptidomimetics (small chains designed to mimic a peptide) and helical foldamers (chain-like molecules that fold into an ordered state in solution). However, synthetic methodologies for the preparation of such molecules remain rather limited and rely most often on [2+2] cycloaddition strategies. A general procedure for preparing β-amino acids is the Michael addition of nitrogen nucleophiles to unsaturated esters, but this had not been developed for cyclobutane derivatives so far.
Angelo Frongia, Università degli Studi di Cagliari, Italy, and colleagues have developed a new tandem amidation/Michael addition protocol to combine a benzoxazolone and cyclobutene-1-carboxylic acid, leading to β-N-heterocyclic cyclobutane carboximide derivatives with a trans structure (pictured below in the center). The team started from cyclobutene-1-carboxylic acid, which was reacted with benzo[d]oxazol-2(3H)-one derivatives and 4-dimethylaminopyridine (DMAP) to obtain β-N-heterocyclic cyclobutane carboximides.
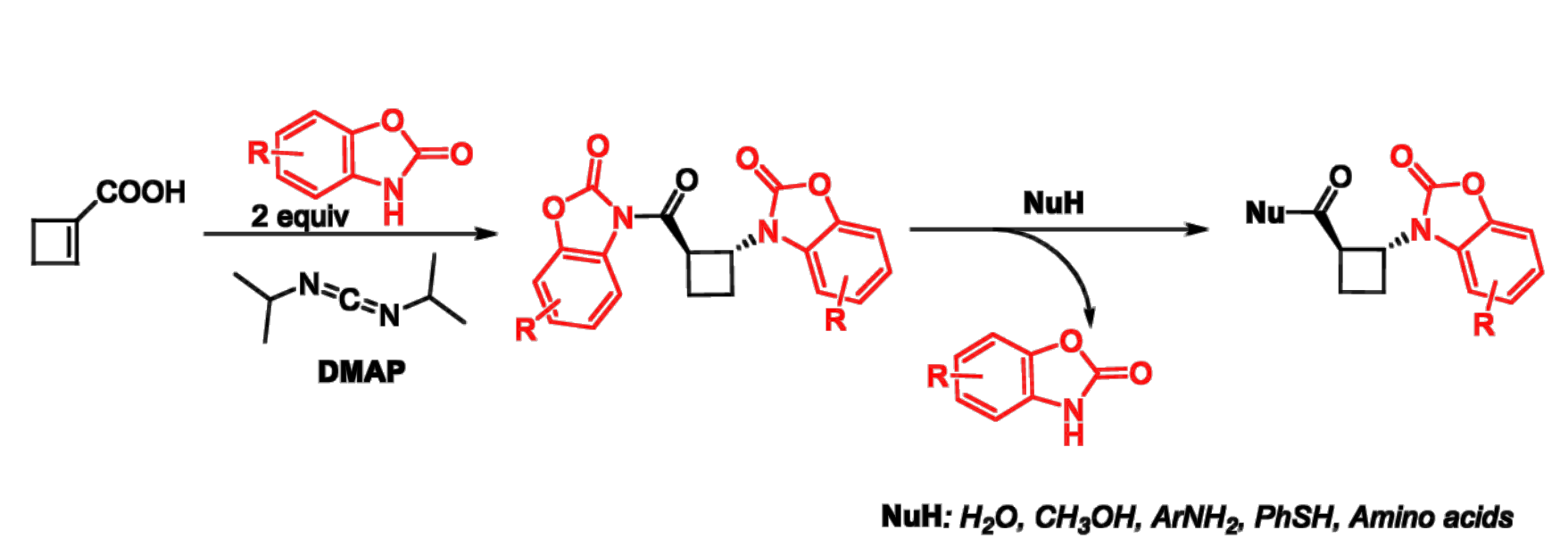
These products can serve as useful intermediates for further transformations since they react easily with various nucleophiles (pictured above on the right). This provides access to diverse derivatives of trans-β-N-heterocyclic cyclobutanecarboxylic acid, including peptidomimetic structures.
- β‐N‐Heterocyclic Cyclobutane Carboximides: Synthesis Via a Tandem Base‐Catalyzed Amidation/aza‐Michael Addition Protocol and Facile Transformations,
Stefano Barranco, Federico Cuccu, Dayi Liu, Sylvie Robin, Régis Guillot, Francesco Secci, Valérie Brenner, Michel Mons, Pierluigi Caboni, David J. Aitken, Angelo Frongia,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300183