Targeted therapies using antibody drug conjugates (ADCs) or peptide drug conjugates (PDCs) can use different physiological phenomena (such as a cellular pH gradient, an intracellular reducing environment, area-specific enzymatic activities, etc.) to release their drug cargo in specific places. Several different linkers that can be cleaved under specific conditions, such as glutathione-sensitive disulfides, have been developed and successfully used in approved ADCs—e.g., in cancer treatments. The development of new types of cleavable linkers could expand the range of applications of this type of treatment.
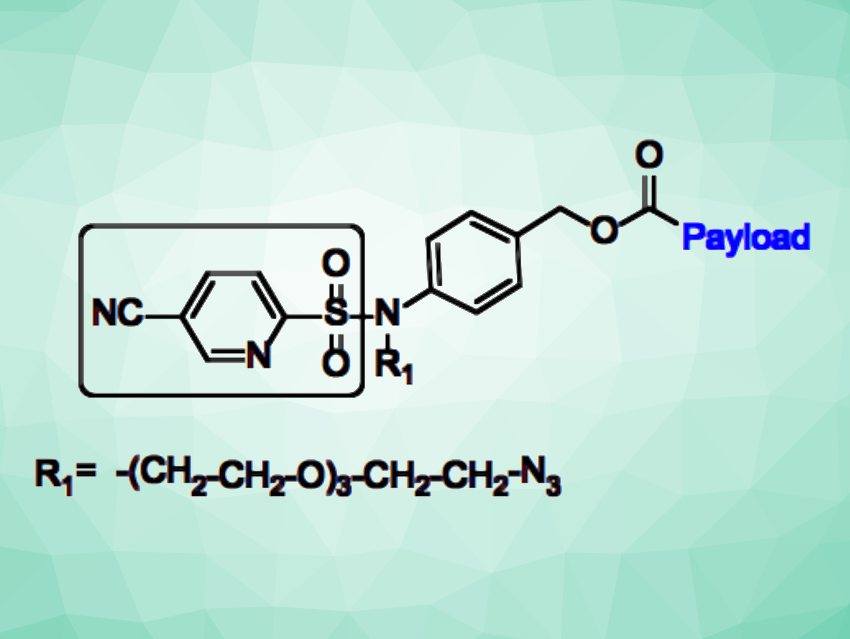
Utpal Majumder, Eisai Inc., Cambridge, MA, USA, and colleagues have investigated whether cellular thiol gradients could serve as a “trigger” for a novel linker design. The team proposed that an electron-deficient arylsulfonamide connected to both the drug payload and the “targeting agent” would react with glutathione or other thiol-containing biomolecules inside cells to trigger the release of the payload. They designed and synthesized a set of electron-deficient arylsulfonamides (example pictured) that were linked to cytotoxic molecules, and then they evaluated their properties.
Since stability in blood circulation and intracellular payload release are the key criteria for a successful linker design, the newly designed linkers were tested for their stability and payload release under physiologically relevant conditions. The most promising sulfonamide-based drug-linker adduct showed acceptable stability in human serum (ca. 10 d). It was bound to an anti-human folate receptor alpha (FRα) antibody, which is an attractive target in oncology, and the intracellular payload release was confirmed using cytotoxicity assays.
According to the researchers, the work outlines the first non-disulfide-based linker that exploits a physiological thiol gradient as a trigger for drug release in the context of ADCs. However, further evaluation of the in–vivo efficacy and pharmacokinetic studies of the new conjugates are still needed.
- A novel concept for cleavable linkers applicable to conjugation chemistry – design, synthesis and characterization,
Utpal Majumder, Xiaojie Zhu, Daniel Custar, Danyang Li, Hui Fang, Sharon McGonigle, Earl Albone, Xin Cheng, Weidong Lai, Amy Siu, Karen Bresciano, Andrew Hart, Maarten Postema,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202400826