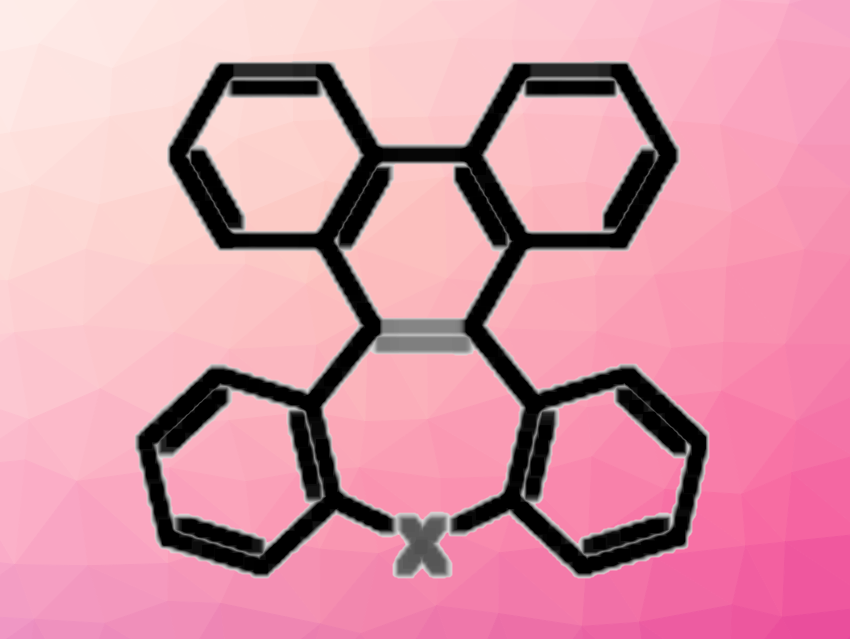
Polycyclic aromatic hydrocarbons (PAHs) can be turned into curved derivatives when non-six-membered rings are embedded in them. The incorporation of heteroatoms can then be used to tune their electronic structure.
Tienan Jin, Tohoku University, Sendai, Japan, and colleagues have investigated dibenzo[g,p]chrysene (DBC) derivatives in which seven-membered rings with heteroatoms are embedded. The target products were synthesized using a tandem oxidative ring expansion. The team started from an o-biphenyl-linked methylenexanthene or a corresponding thioxanthene derivative and used FeCl3·6H2O as an oxidizer in the presence of o-chloranil, with PhCl as the solvent. The reactions were performed at 80 °C.
Under these conditions, dibenzo[b,f]phenanthro[9,10-d]oxepine (DBPO) or dibenzo[b,f]phenanthro[9,10-d]thiepine (DBPT) derivatives were obtained in moderate to high yields. The team proposes that the reaction proceeds via a cycloadduct formed between the alkene moiety of the methylenexanthene derivative and o-chloranil and a ring expansion of a six- to a seven-membered ring as key steps. Further extending this work could lead to new functional π-based molecules.
- Synthesis of Curved Polycyclic Arenes Embedded with Oxepine and Thiepine Using Ring Expansion Strategy,
Sheng Zhang, Hidenori Matsuyama, Itaru Nakamura, Masahiro Terada, Tienan Jin,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01730