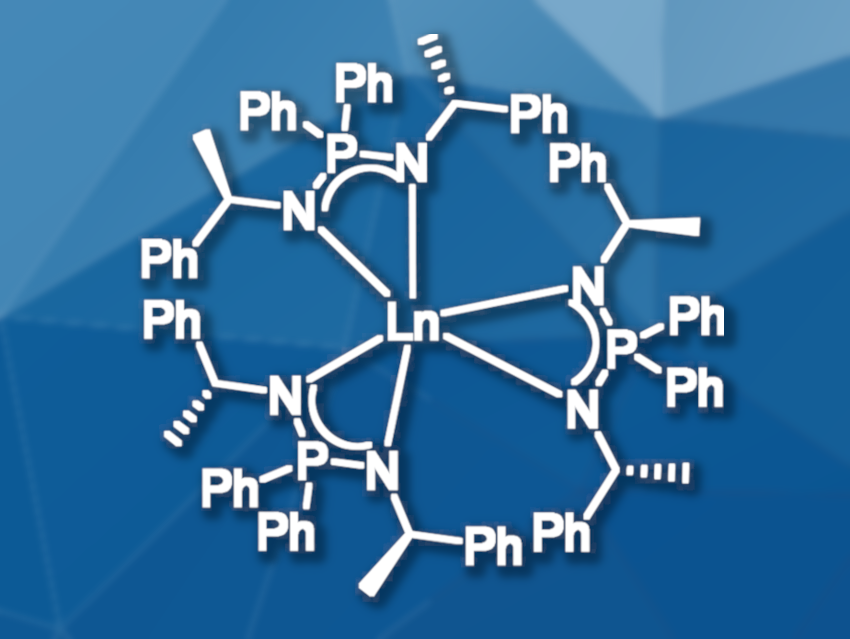
Lanthanide complexes are useful catalysts, e.g., for the polymerization of olefins and for the activation of small molecules like carbon dioxide. Due to their unique luminescence properties, trivalent lanthanide ions are important for many technological applications. Enantiopure lanthanide complexes are especially interesting because of their potential as materials with circularly polarized luminescence. Most of the established lanthanide complexes contain cyclopentadienyl ligands or various N-donating ligands such as amidinates and guanidinates. Iminophosphonamide ligands, which are analogues of the amidinates containing phosphorus instead of carbon, provide different structural features for the resulting complexes and can be easily monitored by NMR.
Peter W. Roesky, Karlsruhe Institute of Technology, Germany, and colleagues have synthesized a series of enantiopure lanthanide complexes with three iminophosphonamide ligands (pictured). The complexes were synthesized from the trichlorides of yttrium, lanthanum, samarium, terbium, and lutetium and the potassium salt of the ligand in refluxing toluene. The air- and moisture-sensitive compounds were analyzed by infrared spectroscopy and elemental analysis, as well as NMR spectroscopy for the diamagnetic complexes. All five complexes were also characterized by single-crystal X-ray diffraction. the presence of three coordinating ligands and the enantiopurity of all five complexes were confirmed.
Sm(III) and Tb(III) are known for their photoluminescence. For the samarium complex, ligand-centered luminescence was observed in addition to the typical emissions of Sm(III), but was relatively short-lived and inefficient. In the case of the terbium complex, the typical luminescence of Tb(III) was enhanced by the coordination of the ligand.
- Homoleptic Enantiopure Lanthanide Complexes: Synthesis, Structure, and Characterization,
Bhupendra Goswami, Xiaofei Sun, Thomas J. Feuerstein, Michael T. Gamer, Peter W. Roesky,
Organometallics 2023.
https://doi.org/10.1021/acs.organomet.2c00603