Main group metal hydrides are interesting research targets in inorganic chemistry. For example, alkali metal pnictogenides (MEH2, M = alkali metal, E = N, P, As) are important starting materials in this field. They provide access to, e.g., a variety of phosphorus and arsenic compounds. The analogous antimony chemistry, however, is less accessible. Potassium antimonide (KSbH2), for example, is unstable, which prevents its isolation and hinders its use as a transfer reagent for SbH2 groups.
Carsten von Hänisch, University of Marburg, Germany, and colleagues have developed an approach for the synthesis and isolation of alkali metal antimonides. The team synthesized antimonides of the type [M(L)xSbH2] (M = Li, Na, K, Rb, Cs; L = pmdta, crown ether; pmdta = pentamethyldiethylentriamine). They started from stibine (SbH3), which was reacted with n-butyllithium and M(hmds) (hmds = hexamethyldisilazane) or MOtBu in the presence of pmdta.
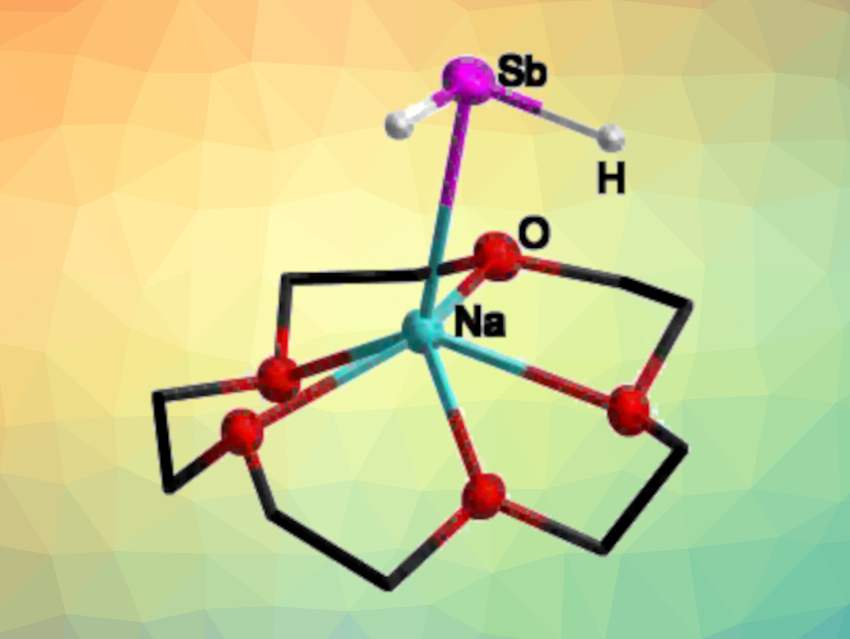
The resulting compounds of the type [Li(pmdta)xSbH2] decompose at temperatures above –50 °C. The use of an appropriate crown ether instead of pmdta allowed the team to isolate the desired alkali metal antimonides in the form [M(crown ether)xSbH2]. These products (example pictured) are stable at room temperature (except for the lithium compound), and were fully characterized. In addition, the researchers prepared the 1,4-dioxane adduct [Na(dioxane)xSbH2], which was used as a starting compound for the synthesis of the first primary silylstibane, (Me3Si)3SiSbH2.
- Synthesis and Application of Alkali Metal Antimonide ‐ A New Approach to Antimony Chemistry,
Kevin Dollberg, Selina Schneider, Roman-Malte Richter, Tobias Dunaj, Carsten von Hänisch,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202213098