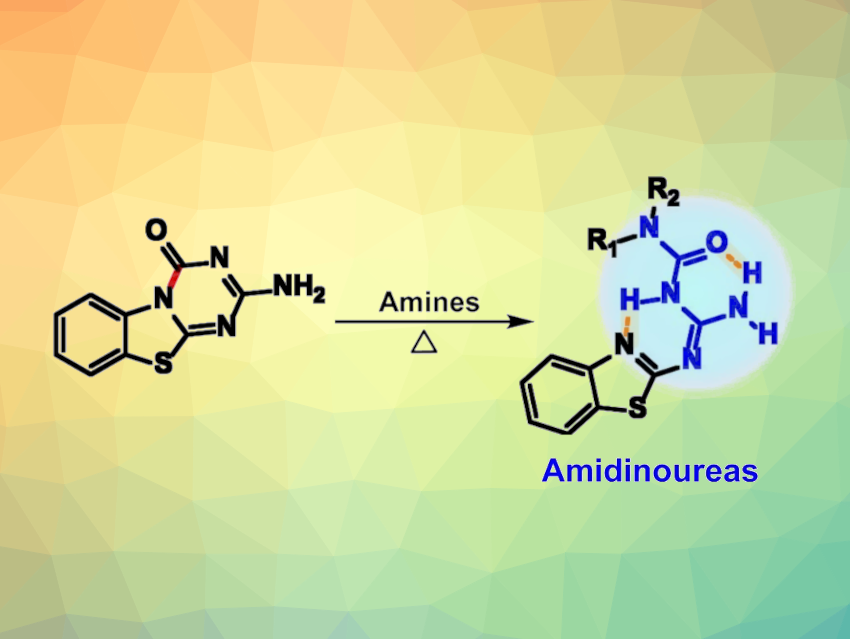
Amidinoureas have unique structural properties and biological activities. The compounds have a scaffold involving seven heteroatoms, combining a urea and an amidine unit. They can be functionalized to give a variety of derivatives, and their structure is interesting in the context of interactions with biomolecular targets. However, despite their apparent simple chemical structure and medicinal potential, amidinoureas have not been well-explored, partly due to a lack of synthetic accessibility.
Stéphane Rocchi, Université Côte d’Azur, Nice, France, Rachid Benhida, Université Côte d’Azur and Mohamed VI Polytechnic University, Ben Guerir, Morocco, Cyril Ronco, Université Côte d’Azur and Institut Universitaire de France (IUF), Paris, and colleagues have developed a method for the preparation of a variety of substituted amidinoureas. The team’s approach is based on an unexpected ring opening of benzothiazolo-1,3,5-triazine-2-ones, resulting in the formation of linear amidinourea derivatives. This observation led the team to a general protocol for the synthesis of amidinoureas via transamination reactions of N-(N-(benzo[d]thiazol-2-yl)carbamimidoyl)aniline-1-carboxamide with different amines.
The team evaluated the activity of the products against melanoma (skin cancer) cells and derived structure–activity relationships. The most active compounds showed antiproliferative activities in the low micromolar range. Some of the products also showed effective anticancer properties in a panel of cell lines of other cancer types. This work might pave the way for further optimization of this family of compounds as anticancer drugs.
- Design, Synthesis and Biological Evaluation of Novel Anticancer Amidinourea Analogues via Unexpected 1,3,5‐Triazin‐2‐one Ring Opening,
Oleksandr Grytsai, Nedra Hamouda-Tekaya, thomas botton, stephane rocchi, rachid benhida, Cyril Ronco,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300493