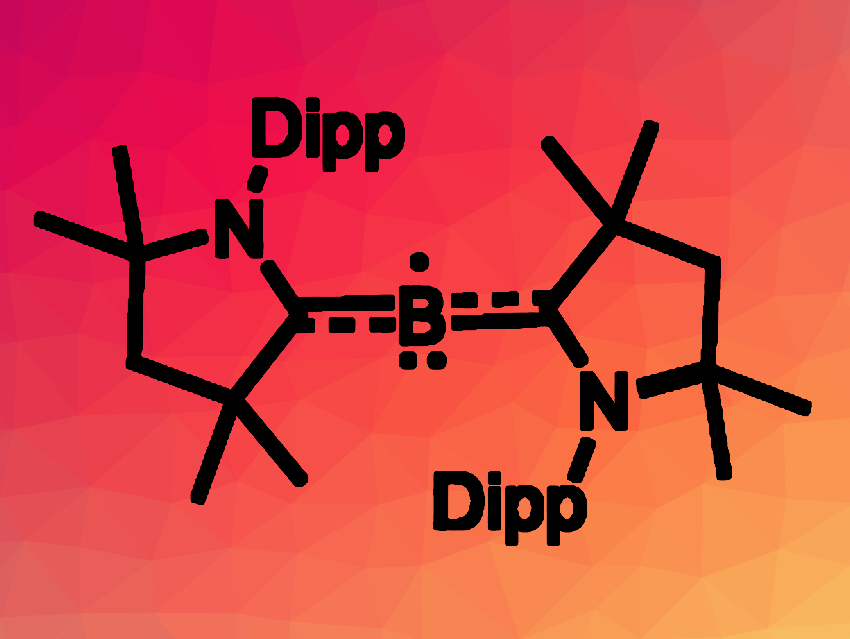
Stable, formally zero-valent complexes of an element can have interesting reactivities. Monoatomic complexes of the type LnE are known for the even-numbered main group elements, e.g., L2Be or L2C. In contrast, paramagnetic group 13 analogues have only been found at low temperatures in matrix isolation studies due to their reactivity. Stable complexes containing formal B(0) centers, for example, contain multiple boron atoms and are diamagnetic, such as diborynes (L→B≡B←L).
Conor Pranckevicius, University of British Columbia, Kelowna, Canada, and colleagues have synthesized the first “bottleable”, fully characterized example of a neutral group 13 atom bound only by neutral donor ligands, (MeCAAC)2B (pictured, Dipp = 2,6-diisopropylphenyl). The team used cyclic (alkyl)(amino)carbenes (CAACs) as ligands to stabilize the complex. They reduced (MeCAAC)BBr3 with KC8 in the presence of additional equivalents of the ligand and obtained the radical (MeCAAC)2B in a yield of 72 %.
The structure of the product was confirmed using X-ray diffraction, which showed an almost linear C–B–C arrangement with B–C bonds that are a little longer than a typical B=C double bond. As expected, the complex is paramagnetic, and according to the researchers, it represents the first isolated example of a neutral, two-coordinate boron radical. The complex is stable in solution under an inert atmosphere. In the solid state, it withstood heating to 150 °C under an argon atmosphere without decomposition.
- Ambient Temperature Isolation of a Monatomic Boron(0) Complex,
William Kennedy, Vignesh Pattathil, YuXiang Wei, Felipe Fantuzzi, Conor Pranckevicius,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.4c14915