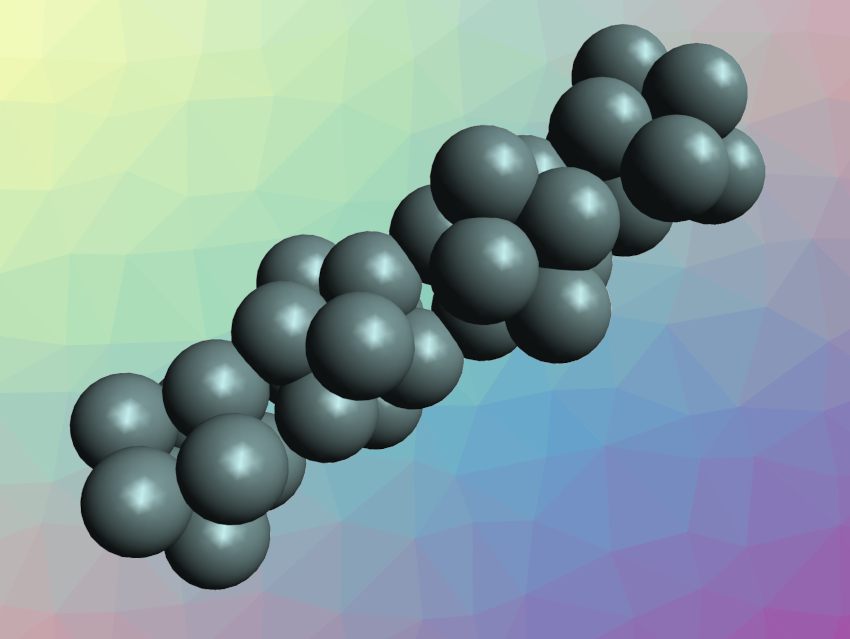
Clusters of atoms often have properties that are different from both single atoms and the corresponding bulk material. The controlled synthesis of materials from such clusters can be challenging. Cluster-assembled tin species, for example, are elusive.
Alexander I. Boldyrev, Utah State University, Logan, USA, Zhong-Ming Sun, Nankai University, Tianjin, China, and colleagues have synthesized the “naked” tin cluster anion Sn368‒. The cluster anion was obtained in form of the salt [K(2,2,2-crypt)]8Sn36 (2,2,2-crypt = 2.2.2-cryptand) via oxidative coupling reactions of Sn94‒ clusters (a type of Zintl ion). The team reacted K4Sn9, dissolved in ethylenediamine, with Na[Zn(C5H5)3] as an oxidant in the presence of 2,2,2-crypt. The product was isolated in the form of green crystals.
The synthesized [K(2,2,2-crypt)]8Sn36 was characterized using single-crystal X-ray diffraction. The team found that the compound contains tetrameric Sn368‒ clusters (pictured), which form a nanorod/nanowire with a length of ca. 2.7 nm. According to the researchers, the cluster is the first example of a tin cluster assembled from Zintl-ion fragments, as well as the first synthesized tin nanorod.
- Sn368‒: A 2.7 nm Naked Aromatic Tin Rod,
Nikolay V. Tkachenko, Wei-Xing Chen, Harry W. T. Morgan, A. Muñoz-Castro, Alexander Boldyrev, Zhong-Ming Sun,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc01745h