There are only a few examples of truly solvent- or ligand-free, “naked” metal ions, in the form of salts of larger weakly coordinating anions (WCAs). In most M[WCA] salts, reactive metal cations M+, such as Ga+, In+, or Cu+, are coordinated by solvent molecules in the solid state, which can be very difficult to remove by simple evacuation. In very weakly coordinating solvents, the “naked” metal ions generally display enhanced reactivity and catalytic activity compared with more “traditional” metal ion sources.
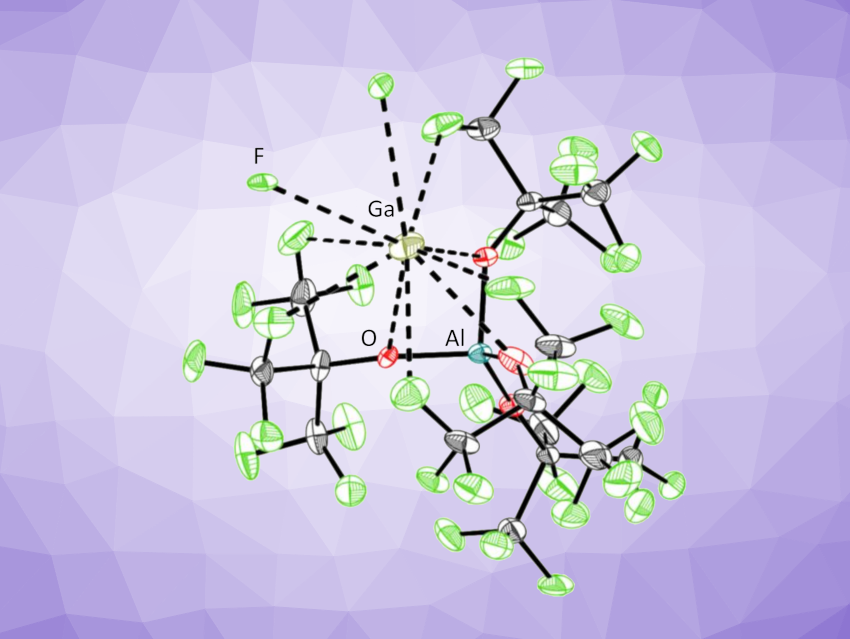
Ingo Krossing, University of Freiburg, Germany, and colleagues have prepared solvent-free Ga[pf] and In[pf] salts ([pf]– = [Al(ORF)4]–; RF = C(CF3)3), which represent very rare examples of salts with truly “naked” metal cations, and analyzed their solid-state structure (example pictured). The team synthesized Ga[pf] via the oxidation of Ga0 with Ag[pf] in 1,2,3-F3C6H3, a very weakly coordinating solvent. In[pf] was prepared by layering a concentrated solution of [In(PhF)2][pf] in 1,2,3,4-F4C6H2 with n-pentane. The researchers obtained single crystals suitable for X-ray diffraction studies for both Ga[pf] and In[pf].
The team found that in Ga[pf], the [pf]– anion acts as a weak, multidentate ligand: The Ga+ ion is weakly coordinated by five fluorine and three oxygen atoms of three –OC(CF3)3 groups, and the fourth –OC(CF3)3 group of each [pf]– anion coordinates to another gallium ion via two F atoms. Density functional theory (DFT) calculations showed that the [pf]− anion interacts only weakly with the Ga cation and that the Ga atom in the salt is barely reduced compared with a truly “naked” Ga+ cation.
The structure of In[pf] contains two crystallographically independent “naked” In+ cations, interacting with four [pf]− anions each, only via the F atoms and without In–O contacts. The structure of In[pf] has a higher ionic character than Ga[pf], and its solid-state packing is determined by the large, almost spherical [pf]− anion, which forms a slightly distorted cubic closed packing with the In+ cations occupying half of the tetrahedral sites.
The team also found that univalent gallium and indium cations are surprisingly stable in solutions of the strongly coordinating solvents MeCN and OEt2. Overall, the work could provide new options for subvalent Ga+ and In+ chemistry.
- On the Synthesis and Structure of ‘Naked’ Ga(I) and In(I) Salts and the Surprising Stability of Simple Ga(I) and In(I) Salts in the Coordinating Solvents Ether and Acetonitrile,
Antoine Barthélemy, Harald Scherer, Hanna Weller, Ingo Krossing,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202400897