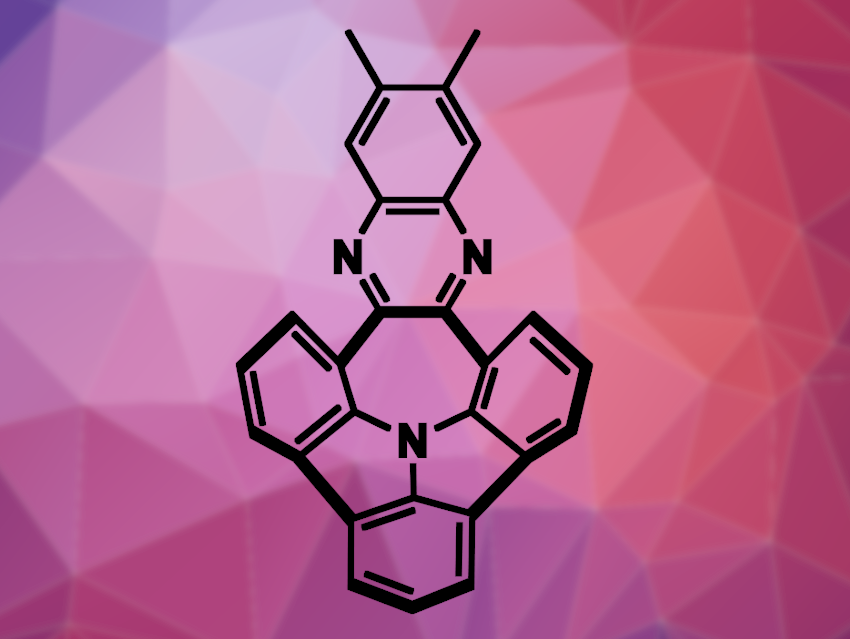
Polycyclic aromatic hydrocarbons (PAHs) can form nonplanar structures, such as buckybowls—bowl-shaped molecules that can be considered fragments of the buckminsterfullerene C60. Bowl-shaped molecules can be useful, e.g., in supramolecular chemistry, where their curvature influences intermolecular interactions. Doping with heteroatoms can be used to modulate the properties of PAHs.
Marcin Lindner, Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Artur Kasprzak, Warsaw University of Technology, Poland, and colleagues have found that certain bowl-shaped, N-doped, fluorescent PAHs (example pictured) can be used as molecular receptors for the recognition of metal ions. The team selected four examples with either two methyl substituents (pictured), two Br substituents, a single Br substituent, or one F substituent. First, they studied the metal ion recognition capabilities of the bowl-shaped PAHs using potentiometric measurements. The researchers found that membranes doped with the doubly methyl-substituted bowl-shaped PAH showed selective ion bonding—for example, they were 350 times more selective for Cs+ over Na+.
The team further investigated the interaction between the PAHs and the ions using fluorescence spectroscopy. When Cs+ was added to the doubly methyl-substituted bowl-shaped PAH in solution, they observed turn-on fluorescence behavior, which they attributed to the formation of a complex between the PAH and the ion. The PAH as a receptor could also recognize other metal ions. According to the researchers, the work provides a new class of molecular receptors for the recognition of metal cations and could lead to new applications for bowl-shaped PAHs.
- Metal cations recognition by bowl-shaped N-pyrrolic polycyclic aromatic hydrocarbons,
Daria Szeląg, Jakub S. Cyniak, Joachim Ażgin, Jakub Wagner, Marcin Lindner, Wojciech Wróblewski, Artur Kasprzak,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC02586E