Molecular switches can be used to change the properties of a material upon an external stimulus such as, for example, light. Sterically crowded alkenes with bulky substituents can be useful in this context. They can show chirality around the central double bond and allow for responses to light, heat, or electrochemical stimuli. The use of planar chiral building blocks, such as paracyclophanes, rigid cycloalkenes, bridged annulenes, or disubstituted ferrocenes is underexplored in the development of chiral switches. In addition to chirality, the redox activity of ferrocene derivatives makes them interesting building blocks for the construction of multi-stimuli-responsive systems.
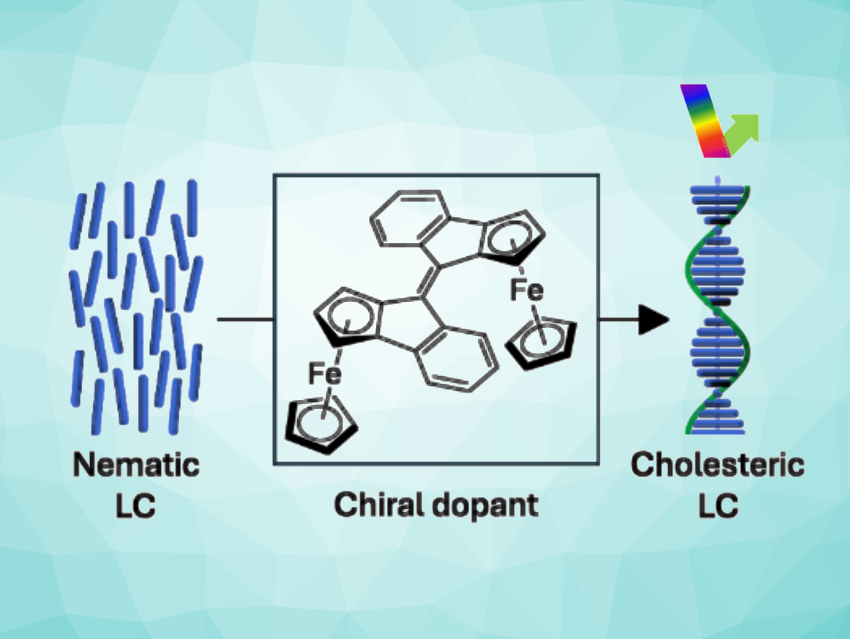
Ben L. Feringa, University of Groningen, The Netherlands, and colleagues have synthesized multi-stimuli-responsive dimers of an enantiopure planar chiral ferrocene-indanone building block (pictured). The dimer can undergo thermal or light-induced E/Z isomerization and can also be reversibly and quantitatively oxidized to both a monocationic and a dicationic state. It can act as a chiral switch and be used, e.g., as a dopant in liquid crystals to allow a change in their properties upon an external stimulus.
The team started from commercially available 2-bromobenzoyl chloride, which was reacted with ferrocene in a Friedel-Crafts reaction, followed by a Pd-catalyzed intramolecular coupling using a chiral ligand to close the five-membered ring in an enantioselective manner. A reaction with Lawesson’s reagent was then used to introduce a thioketone group, followed by dimerization to give the desired chiral products. During the final dimerization step, the relative orientation of the ferrocene motifs (syn), the double bond geometry (E), and the helicity of the switch are fixed, and only a single switch isomer is formed from a particular enantiomeric precursor.
The synthesized switch can be used as a dopant in nematic liquid crystals (pictured above on the left). Its addition led to the formation of a cholesteric LC phase (pictured above on the right). This helically organized phase reflects light of a certain color selectively. The team found that redox reactions of the molecular switch can be used to tune the liquid crystal’s reflection color by up to 84 nm: The initial blue color of doped liquid-crystal droplets suspended in water changes to green upon oxidation of the dopant. The switch might also be useful in other applications where (redox) chiroptical switching is required.
- A Multiresponsive Ferrocene‐Based Chiral Overcrowded Alkene Twisting Liquid Crystals,
Maximilian Fellert, Robert Hein, Alexander Ryabchun, Yohan Gisbert, Charlotte N. Stindt, Ben L Feringa,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202413047