Transition-metal catalysts can be used, e.g., for hydroboration reactions in organic chemistry. Many of the metals used in transition-metal catalysis are rare and/or expensive, which can hamper sustainability and accessibility. “Using cheap metals for noble tasks” would be a preferable alternative—magnesium, for example, is Earth-abundant, biocompatible, and inexpensive.
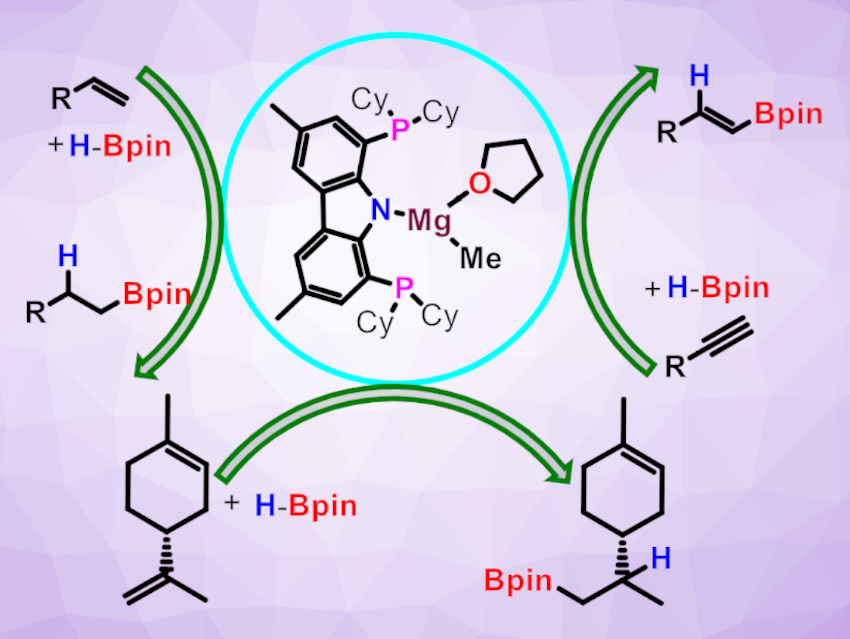
Rajesh G. Gonnade, Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, and CSIR-National Chemical Laboratory, Pune, India, Debasis Koley, Indian Institute of Science Education and Research (IISER), Kolkata, India, Sakya S. Sen, CSIR-National Chemical Laboratory and AcSIR, and colleagues have prepared a bis(phosphino)carbazolido ligand and reacted it with Grignard reagents to access the corresponding monomeric magnesium complex (pictured above).
The ligand was prepared starting from di-p-tolyl amine, which was tetrabrominated and then lithiated using nBuLi, followed by a reaction with chlorodicyclohexylphosphine (PCy2Cl). The resulting PNP-donor ligand was reacted with MeMgBr in tetrahydrofuran (THF) to obtain the desired catalyst.
The resulting bis(phosphino)carbazolido-magnesium-methyl complex can catalyze the hydroboration of alkenes, alkynes, and biologically important terpenes using pinacolborane (HBpin, example reactions pictured). Such magnesium-catalyzed hydroboration reactions could provide an attractive path to borane-functionalized hydrocarbons, which have uses in cross-coupling reactions.
- Monomeric Magnesium Catalyzed Alkene and Alkyne Hydroboration,
Rohit Kumar, Sayan Dutta, Vishal Sharma, Praval P Singh, Rajesh Gonnade, Debasis Koley, Sakya S. Sen,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201896