Fluorophores that can show different fluorescence colors can be useful, e.g., for multicolor displays or anti-counterfeiting applications, especially if they can be used in the solid state. Switching between fluorescence colors can be due to, for example, mechanochromism, in which the color changes under mechanical stimuli such as grinding or pressing, and the change can often be reverted by heating or fuming with organic solvents. Another type of fluorescence switching is acidochromic luminescence, in which the color changes upon protonation/deprotonation.
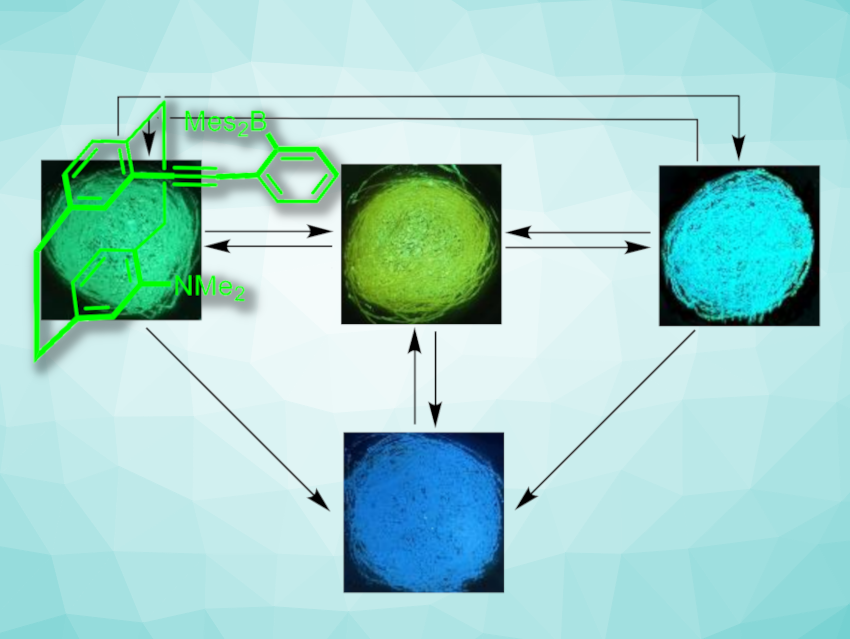
Cui-Hua Zhao, Shandong University, Jinan, China, and colleagues have synthesized a [2.2]paracyclophane-based triarylborane (pictured in green) whose fluorescence in the solid state can be switched between four colors (pictured). The molecule, which the team calls g-BPhANMe2-Cp, consists of a [2.2]paracyclophane unit that is substituted with an electron-accepting (2-dimesitylboryl)phenyl]ethynyl group and an electron-donating dimethylamino group. It was synthesized from a iodo- and NMe2-substituted [2.2]paracyclophane precursor via a Sonogashira coupling with (2-bromophenyl)acetylene, followed by lithiation and a borylation using dimesitylborylfluoride.
The product shows green fluorescence, which can be shifted towards yellow by grinding. The change can be reverted by heating to 120 °C. Upon fuming the ground powder with organic solvents such as dichloromethane, the fluorescence color changed to cyan. The change can be reverted by further grinding. In addition to these three colors that can be achieved using mechanochromic effects, the product also shows acidochromism. When it was brought into contact with trifluoroacetic acid (TFA) fumes, the fluorescence color changed to blue (pictured above in the bottom row). From there, the form with yellow fluorescence can be obtained by fuming the protonated sample with triethylamine (TEA).
The fluorescence efficiency of the molecule in the solid state remains high through all of these changes. According to the researchers, the work provides a rare example of a single-component fluorophore displaying solid-state multicolor fluorescence switching.
- The Solid‐state Multi‐color Fluorescence Switching from a [2.2]Paracyclophane‐based Triarylborane,
Lian-Feng Guo, Min Wang, Cui-Hua Zhao,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202402287