Molecular switches can reversibly change between two or more forms upon an external stimulus. This can be useful, e.g., in medicinal chemistry or sensing. Often, an E/Z-isomerization is the reaction responsible for the switching, generally in an alkene, imine, or azo group. Phosphaalkenes can also undergo this type of isomerization, but are less well explored.
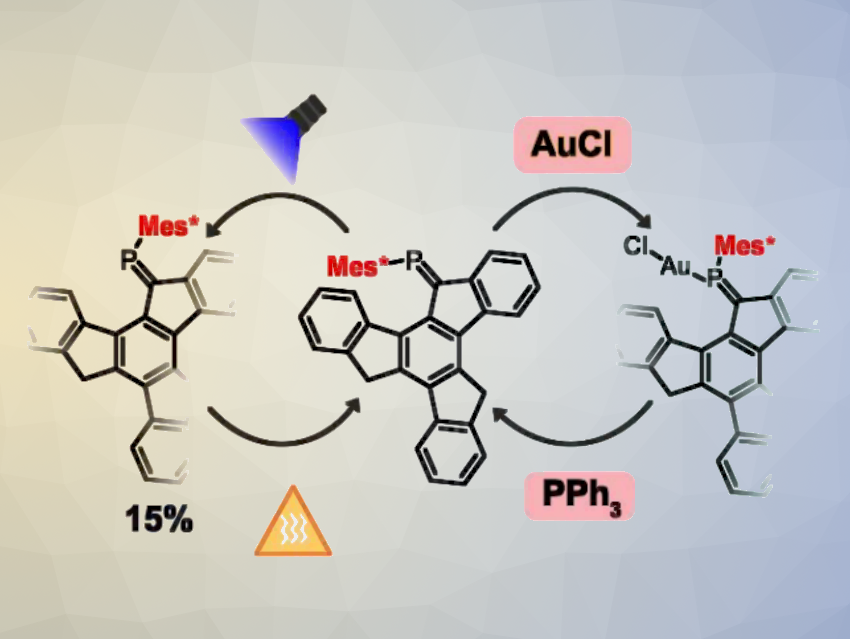
Stefano Crespi, Andreas Orthaber, Uppsala University, Sweden, and colleagues have found that a polycyclic aromatic hydrocarbon functionalized with a phosphaalkene unit can undergo molecular switching based on a variety of molecular triggers, including light irradiation, metal coordination, and deprotonation. They used a compound with a polycyclic truxene (TruxC) core of the type TruxC=P-Mes* (pictured, Mes* = 2,4,6-tris-t-butyl-benzene). The team found that upon the complexation of AuCl, the compound undergoes a Z to E isomerization. PPh3 can be used to induce decomplexation and revert the isomerization.
In addition to the metal-induced switching, the molecule can also act as a photoswitch, undergoing an incomplete isomerization under light irradiation. Another option is the formation of an anionic derivative: When the compound in its Z-isomer form was reacted with KC8, a dianionic species with an E configuration at the phosphaalkene unit was obtained. Overall, the work demonstrates a new strategy for building molecular switches.
- Reversible Metal‐Mediated Molecular Switching of a Phosphaethene Polyaromatic Hydrocarbon,
Toma Bhowmick, Jordann AL Wells, Mohd Asif Ansari, Jorn D. Steen, Stefano Crespi, Andreas Orthaber,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403974
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


