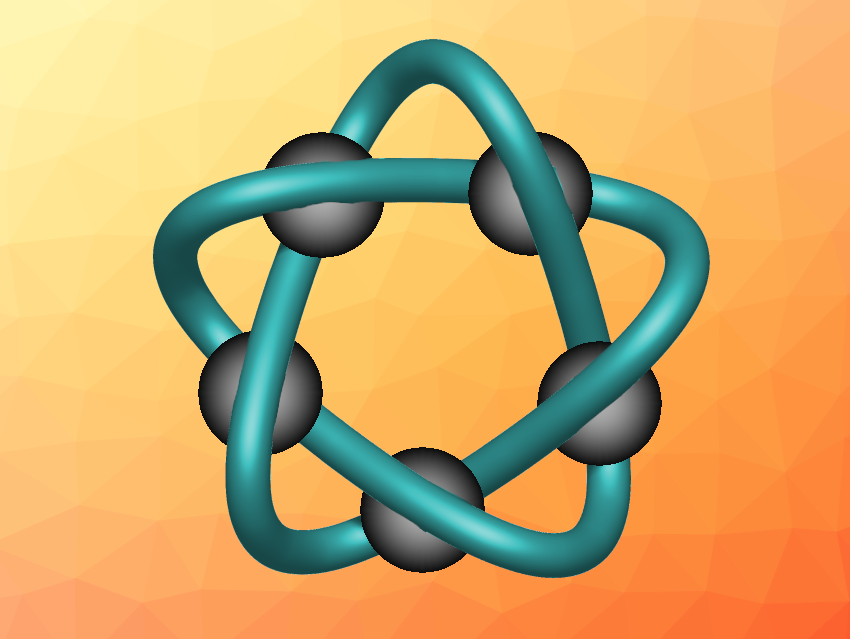
Molecular knots can have interesting structures and useful functions. Most often, they are synthesized using metal ions as a template via coordination chemistry. The resulting knotted organic molecules are chelating ligands, which could also coordinate other metal ions than those originally used for templating. This may lead to interesting species, such as complexes with coordinatively unsaturated metal sites that are relevant, e.g., in catalysis or molecular recognition.
Xiao-Yu Cao, Xiamen University, China, Zhi-Hui Zhang, Liang Zhang, East China Normal University, Shanghai, China, and colleagues have prepared an organic molecule in the form of a cinquefoil knot (simplified structure with bound metal ions pictured) with a strand length of 140 atoms, using copper(II) ions as a template for the synthesis and five ligand monomers as building blocks for the knot. The ligand monomers contain three pyridine units and two thiazole units each. This “3+2” dentate structure of the ligand monomers allows them to form a pentameric complex with five Cu(II) ions, each in a five-coordinated environment. The ligand monomers were then connected via ring-closing metathesis reactions to “close” the knot. The copper can be removed from the knot using tetrasodium ethylenediaminetetraacetate (Na4EDTA), releasing the free “knotted” ligand.
The researchers investigated the reaction of this chelate ligand with other metal ions. They found that a reaction with Zn(OTf)2 (zinc triflate) gave a cinquefoil knot that coordinates five zinc(II) ions. This complex was characterized using single-crystal X-ray diffraction, which showed that each Zn(II) ion is coordinated to five nitrogen donor atoms in the knot, with a triflate as a weakly bound sixth ligand. This structure could allow for a tunable, dynamic coordination number at the zinc ions. They can be coordinatively unsaturated metal centers with five ligands and a quasi-free sixth site. Alternatively, the sixth binding site could be used to introduce other ligands and change the properties of the complex.
- Cinquefoil Knot Possessing Dynamic and Tunable Metal Coordination,
Qi Zhou, Xue Dong, Guanyu Chi, Xiao-Yu Cao, Ningjin Zhang, Shitao Wu, Yanhang Ma, Zhi-Hui Zhang, Liang Zhang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c05376