Polycyclic heteroarenes that contain thiophene, furan, or pyrrole rings can have applications, e.g., in optoelectronics. Existing methods for the synthesis of naphthannulated benzoheteroarenes, a class of tetracyclic heteroarenes, mainly
focus on derivatives having a terminal thiophene ring, while approaches to the preparation of compounds with internal thiophene, furan, or pyrrole units are limited in comparison.
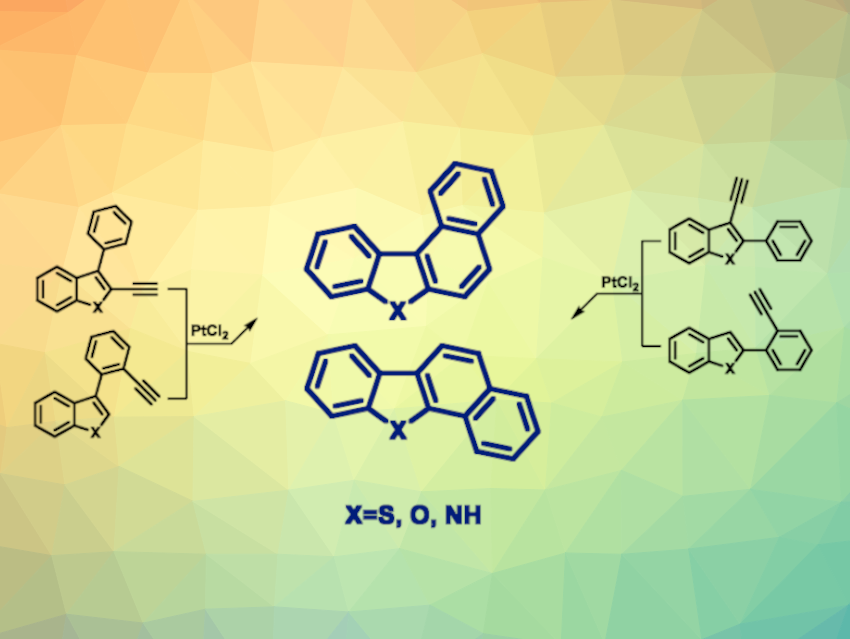
István Jablonkai, Gábor London, HUN-REN Research Centre for Natural Sciences, Budapest, Hungary, and colleagues have developed a method for the synthesis of tetracyclic naphthannulated benzoheteroarenes with thiophene, furan, or pyrrole rings as the heterocycle. The team used a PtCl2-catalyzed 6-endo cyclization of ethynylbiaryls to obtain the desired products. The resulting isomer was controlled by using precursors with the ethynyl subunit in different positions (general structures pictured).
Using this approach, the desired products were obtained in generally high yields. For thiophene and furan-containing molecules, there was a side reaction for precursors in which the alkyne group is attached to the benzene unit of the biaryls, which the team attributes to a competing 5-exo cyclization reaction. The side products are formed in small quantities, but can be challenging to separate. The insights provided by the work could be helpful in the design of suitable ethynylbiaryl precursors for different target compounds.
- Modular Synthesis of Tetracyclic Heteroarenes via Platinum‐Catalyzed Cyclization of Ethynylbiaryl Precursors,
István Jablonkai, Marcell M. Bogner, Barnabás Zsignár-Nagy, József Simon, Gabor London,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202401114