Nitrogen-containing heterocycles are often parts of compounds used in, e.g., pharmaceutical chemistry or materials. Fluorine atoms can be useful in tuning the properties of drug candidates, for example. However, while ring-fluorinated azaheterocycles with one or two nitrogen atoms are well-studied, fluorinated triazaheterocycles have remained challenging to synthesize and study.
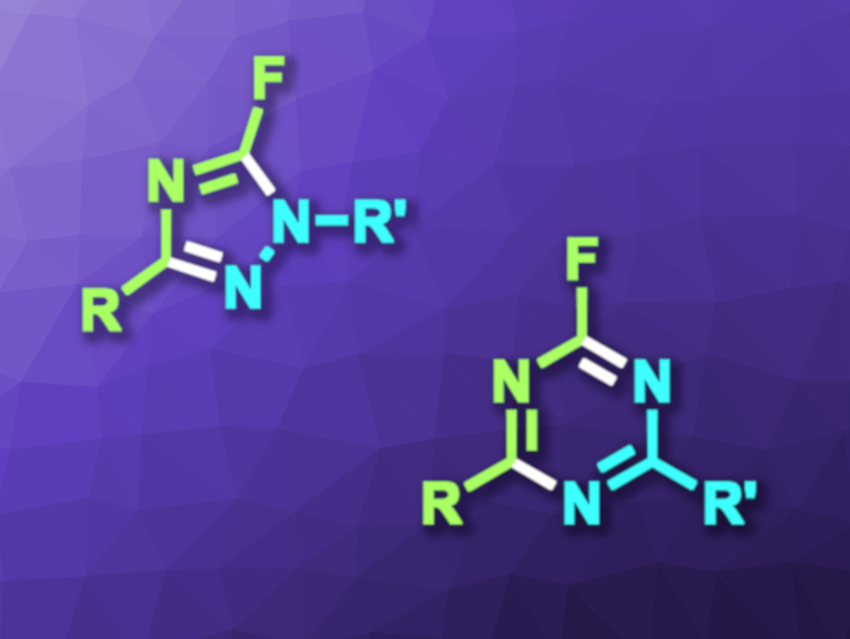
Mang Wang, Northeast Normal University, Changchun, China, and colleagues have developed a method for the synthesis of monofluorinated 1,2,4-triazoles and 1,3,5-triazines (general structure pictured) that is modular and proceeds under metal-free conditions. The team used N-CF3 imidoyl chlorides (R–C(=NCF3)–Cl) as building blocks and reacted them with either hydrazine derivatives or amidines to obtain the desired five- or six-membered rings, respectively.
The synthesis of 5-fluoro-1,2,4-triazoles from hydrazines was performed using triethylamine as a base and dichloromethane (DCM) as the solvent, and 2-fluoro-1,3,5-triazines were obtained in acetonitrile. The reactions were performed at room temperature and under air. The desired products were obtained in mostly high yields. Overall, the work shows the utility of N-CF3 imidoyl chlorides in annulation reactions to prepare fluoroheterocyclic compounds.
- Modular Synthesis of Monofluorinated 1,2,4-Triazoles/1,3,5-Triazines via Defluorinative Annulations of N-CF3 Imidoyl Chlorides,
Chi Gao, Ru Zhong Zhang, Mang Wang,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.5c00300