Acridines, a class of heterocyclic compounds characterized by three fused six-membered rings with a planar aromatic surface, play an important role in natural products, pharmaceuticals, molecular probes, and optoelectronic devices. However, conventional synthesis methods often require specialized starting materials, anaerobic and moisture-free conditions, and multi-step processes for functional group installation.
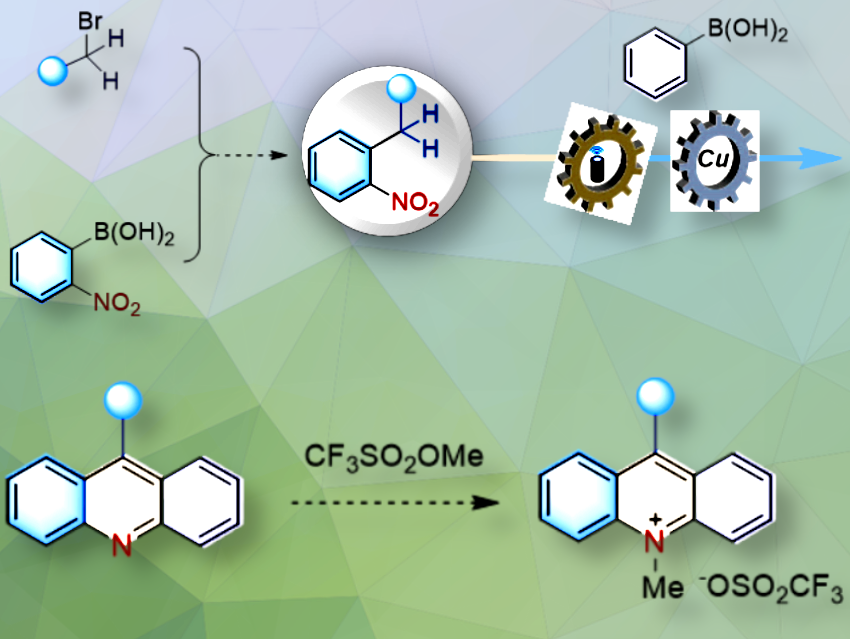
Yu-Mei Lin, Xiamen University, China, and colleagues have developed an innovative and modular approach to synthesizing acridine derivatives, leveraging the synergistic combination of photo-excitation of o-alkyl nitroarenes combined with copper-mediated cascade annulation. This method simplifies the synthesis of diverse acridine compounds and expands the range of possible structures, including unsymmetric and multi-substituted derivatives.
The team began with photo-excitation of o-alkyl nitroarenes, then proceeded through intramolecular hydrogen atom transfer and oxygen relocation to create key amine intermediates. A copper-induced cascade, including Chan-Lam amination, Friedel-Crafts acylation, and aromatization, then completes the one-pot formation of acridine derivatives. The o-alkyl nitroarenes precursors are easily obtained via coupling alkyl halides with o-nitroaryl boronic acids.
The synthesized acridinium salts show extraordinary oxidative strength in their excited states, with reduction potentials from 2.08 to 3.15 V, outperforming many known photocatalysts. This characteristic makes them particularly effective at catalyzing oxidative transformations, making them promising candidates for photochemical reactions.
The researchers state that their work is a significant advance in acridine chemistry, offering a versatile and efficient method for synthesizing structurally diverse acridine compounds. The ease of incorporating substitutions in precursors and the exceptional properties of the resulting acridinium salts open new possibilities for developing advanced photocatalysts and pharmaceuticals.
- Modular Assembly of Acridines by Integrating Photo-Excitation of o-Alkyl Nitroarenes with Copper-Promoted Cascade Annulation,
Haichao Huang, Yifan Jiang, Wei Yuan, Yu-Mei Lin,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202409653