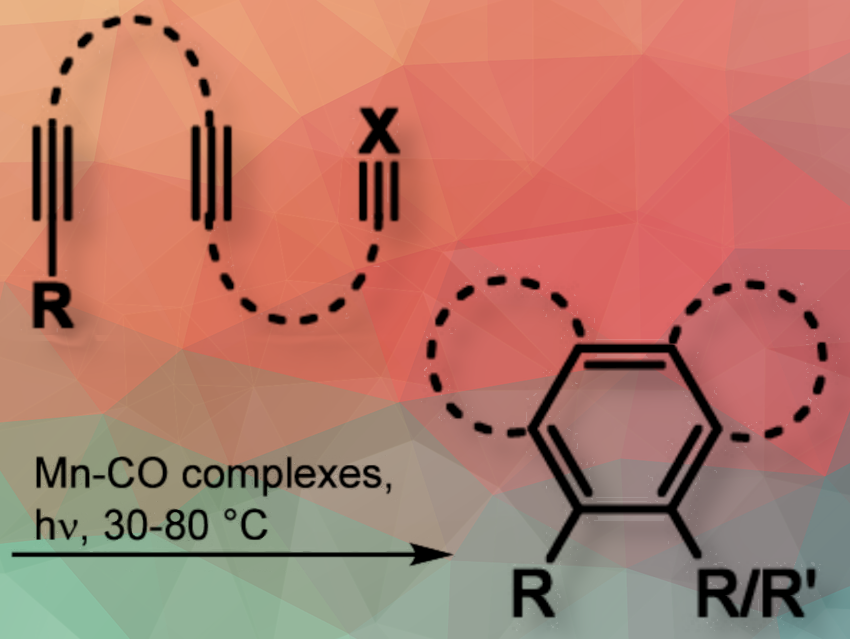
Angelika Brückner and Haijun Jiao, Leibniz Institute for Catalysis e.V. (LIKAT), Rostock, Germany, Marko Hapke, Johannes Kepler University Linz (JKU), Austria, and LIKAT, and colleagues have described the first use of the manganese(I)–carbonyl complex MnBr(CO)5 as a catalyst for the [2 + 2 + 2] cycloaddition of functionalized triynes under mild photochemical conditions using irradiation at 450 nm wavelength to efficiently produce aromatic reaction products without the need for additional photo(redox) catalysts.
The team foud that the introduction of the chelating ligand 1,1-bis(diphenylphosphino)methane (dppm) to form the precatalyst MnBr(CO)3(dppm) significantly improves performance. Presumably, it modifies the catalyst system toward a higher cyclization rate and longer activity, preventing early deactivation as can be presumed for the ligand-free system only using MnBr(CO)5 as a precatalyst. The dppm ligands are only required for the fast generation and the stabilization of the catalytically active “MnBr(CO)3” fragment but are not involved in the catalytic cycle. The catalytic cycle proceeds via oxidative cyclization, insertion of the third alkyne moiety to form a manganacycloheptatriene intermediate, followed by final ring closure through reductive elimination, resulting in the aromatic product.
Reactions are typically carried out at 30 °C, but increasing the temperature to 80 °C optimizes yields for some substrates, even with larger substituents.
The method also supports the synthesis of oligoalkynes from simple building blocks with various functional groups (e.g., substituted aryl, indenyl, Bpin, SiMe3, PPh2, pyridyl, thienyl). This facilitates the preparation of highly substituted benzene derivatives, many of which are suitable for further derivatization via cross-coupling reactions. The cyclization reactions can be extended to hexaynes, yielding biphenyl products through a 2-fold cyclization. Under stoichiometric conditions, the transformation of diynes with phosphaalkynes led to the first photomediated synthesis of phosphinines under rather mild conditions.
- Photochemical Manganese-Catalyzed [2 + 2 + 2] Cycloaddition Reactions,
Benedikt N. Baumann, Phong Dam, Jabor Rabeah, Christoph Kubis, Angelika Brückner, Haijun Jiao, Marko Hapke,
ACS Catal. 2025, 15, 5718–5730.
https://doi.org/10.1021/acscatal.5c00349