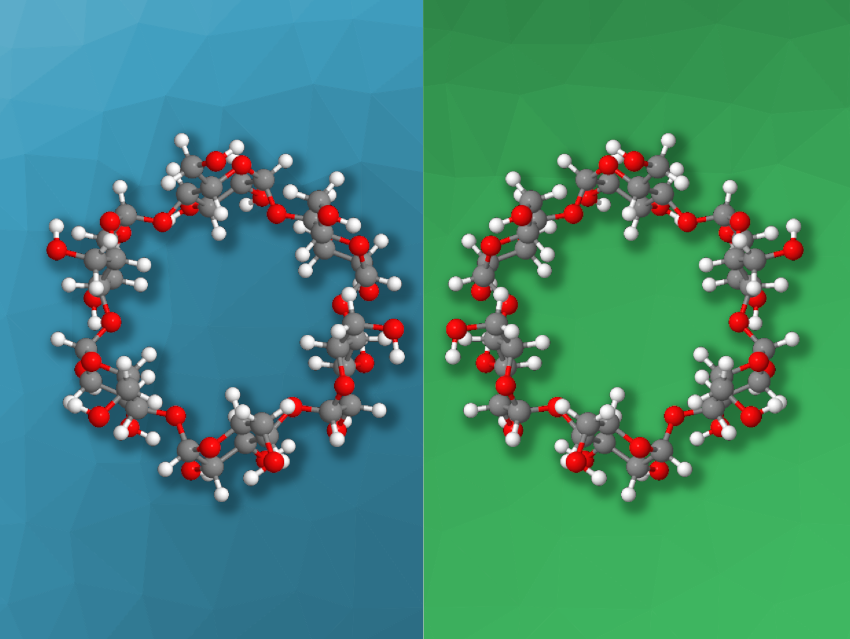
Cyclodextrins (CDs) are macrocyclic oligosaccharides. They can form host-guest complexes and have possible uses, e.g., in supramolecular chemistry, molecular machines, catalysis, or molecular recognition. Native CDs consist of α-1,4-linked D-glucopyranose units, forming a series of macrocycles with six, seven, or eight units (called α, β, and γ-CDs, respectively). These cyclodextrins are inherently chiral, and mirror-image α, β, and γ-L-cyclodextrins have also been prepared.
Daniel W. Armstrong, University of Texas at Arlington, USA, and colleagues have performed a separation of enantiomeric CDs and studied the behavior of mirror-image CDs. The team performed enantiomeric separations using liquid chromatography with optimized solvents and a stationary phase containing macrocyclic glycopeptide chiral selectors. The naturally occurring D-CDs elutes before their mirror-image L-CDs in all cases (i.e., for α, β, and γ-CDs).
The team was able to determine the absolute configurations of the CDs using vibrational circular dichroism (VCD), and they state that D– and L-cyclodextrins are the largest molecules to be completely analyzed and characterized by VCD in terms of absolute configuration. The also studied the enzymatic degradation of D-CDs and L-CDs by four different enzymes and found that synthetic L-CDs were resistant to all four enzymes. The researchers also used the mirror-image CDs as chiral selectors for the enantiomeric separation of small molecules such as amino acids by capillary electrophoresis.
- Actions and Interactions of Mirror-Image Cyclodextrins,
Daniel W. Armstrong, Saba Aslani, Jordan Nafie, Yong Wu, J. Fraser Stoddart,
JACS Au 2024.
https://doi.org/10.1021/jacsau.4c00962