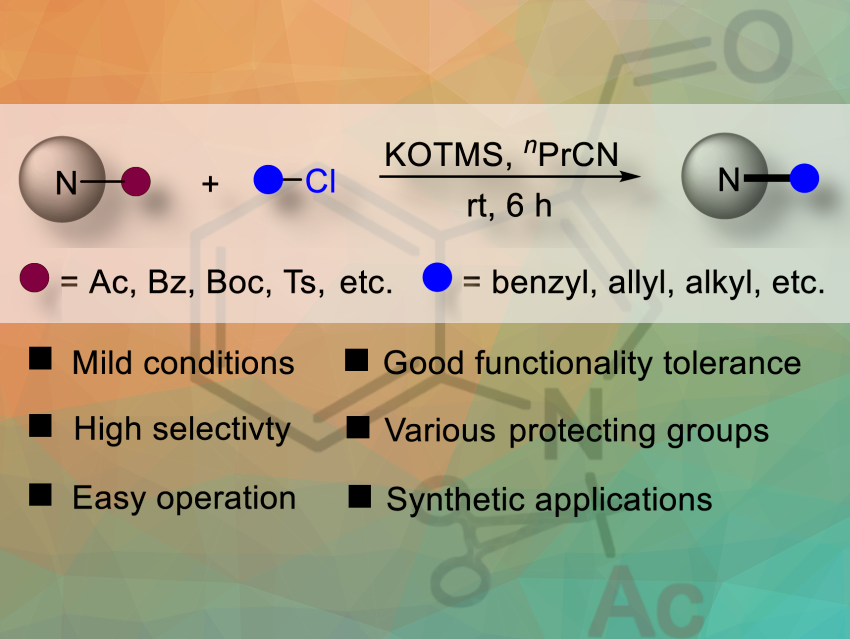
In organic chemistry, protecting groups are used to control reactivity and prevent unwanted side reactions. However, their removal often requires harsh conditions, limiting their usefulness.
Feng Zhao and colleagues, Hunan University of Medicine, Huaihua, China, have develoed a mild and versatile potassium trimethylsilanolate/butyronitrile (KOTMS/PrCN) reaction system for the deprotection and further functionalization of N-heterocycles in a single step at room temerature. A range of N-heterocycles, attached to various protecting groups, including acetyl, benzoyl (Bz), tert-butoxycarbonyl (Boc), and sulfonyl, can be selectively deprotected under mild conditions. Various sensitive functional groups, such as vinyl, halogens, nitro, and cyano, are well tolerated with this system.
The team used 1-acetyl-1H-indole-3-carbaldehyde and benzyl chloride as the model substrates to gain the
optimal reaction conditions.The highest yield was achieved using PrCN as solvent. The team demonstrated that under optimized KOTMS/PrCN conditions, a broad range of benzyl chlorides—bearing various electron-donating, electron-withdrawing, or sterically demanding groups—underwent efficient N-deacetylation and benzylation, yielding products in 88–99%.
The protocol features high efficiency, simple operation, and broad synthetic application. Mechanistic studies suggest a sequential deprotection–benzylation pathway via a N-deacetylated intermediate. Detailed mechanistic investigations and further applications of this mild reaction system is underway in their laboratory, the researchers say.
- Deprotection and Functionalization of Protective N-heterocycles under Mild Conditions,
Wenjing Liu, Zixuan Tan, Qianyu Zhang, Feng Zhao,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202500165