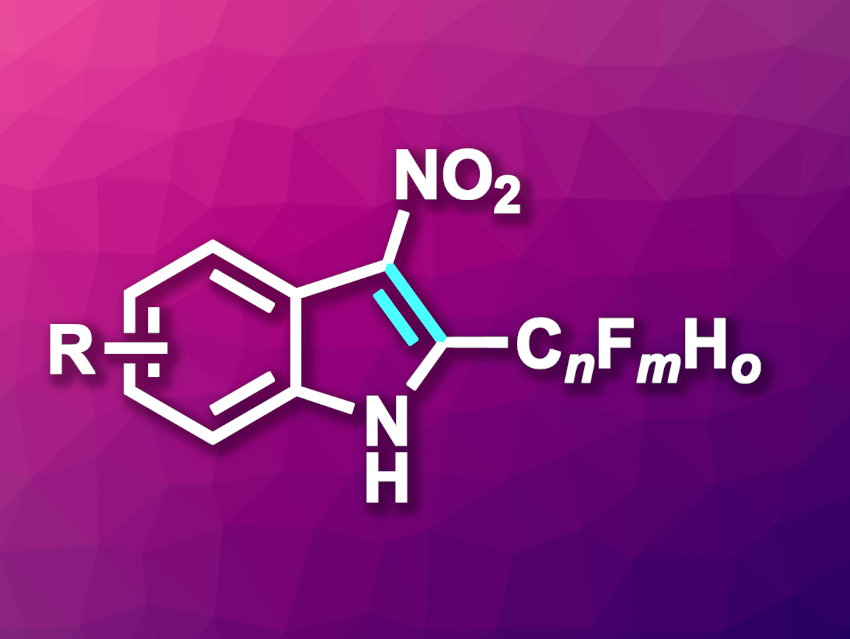
Indoles, aromatic heterocycles composed of a pyrrole ring fused to a benzene unit, are often found, e.g., in natural products or form the core structure of pharmaceutically active compounds. Metal-free methods for their synthesis can be particularly useful in medicinal chemistry. In addition, the introduction of fluoro substituents can often be used to tune the properties of a drug candidate, e.g., their lipophilicity.
Andrey F. Asachenko, Russian Academy of Sciences, Moscow, and colleagues have developed a metal-free method for the synthesis of 3-nitro-2-(per)fluoroalkyl indoles (general product structure pictured) form easily available N-acyl amides. The reaction proceeds via a base-catalyzed intramolecular cyclization. The team converted a wide range of amide precursors using 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) as a base and 1,4-dioxane as the solvent. The reactions were performed at 100 °C.
Under these conditions, the desired functionalized indoles were obtained in high to excellent yields. The researchers demonstrated the scalability of the approach by performing a gram-scale reaction with a yield of 93 %. The method can be used for the introduction of, e.g., HCF2, CF3, C2F5, or C3F7 substituents at the 2-position of the nitro-indoles and has a good functional group tolerance and a broad substrate scope.
- Metal-Free Synthesis of 2-(Per)Fluoroalkyl-3-Nitro Indoles via Intramolecular Cyclization of Amides,
Grigorii K. Sterligov, Maria A. Rasskazova, Egor A. Drokin, Dilshodakhon K. Isaeva, Alexandra A. Ageshina, Sergey A. Rzhevskiy, Olga V. Shurupova, Maxim A. Topchiy, Lidiya I. Minaeva, Andrey F. Asachenko,
J. Org. Chem. 2024.
https://doi.org/10.1021/acs.joc.4c01430