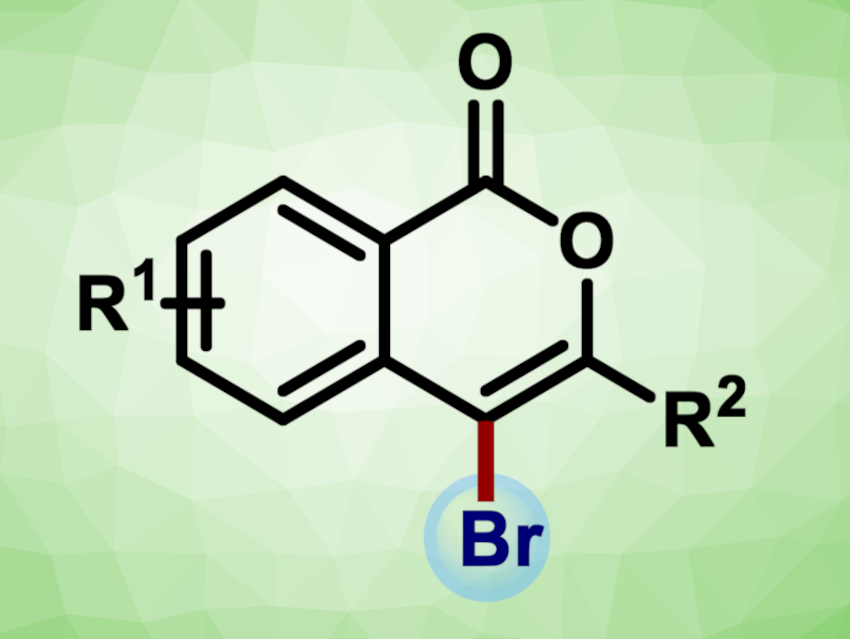
The isocoumarin skeleton is a common structural unit in natural products and biologically active compounds. Developing efficient protocols for accessing isocoumarins is an interesting research target. In addition, the introduction of halogen substituents into the products could provide a handle for further functionalization.
Sermadurai Selvakumar, Indian Institute of Technology Indore, India, and colleagues have developed a sustainable pathway to synthesize 4-bromoisocoumarins via the brominative annulation of 2-alkynylaryloate esters (pictured below). The team used a combination of the hypervalent iodine reagent 4-Cl-HTIB (hydroxy(tosyloxy)4-chloroiodobenzene) as an oxidant and potassium bromide as a halogen source. The reactions were performed at room temperature over 6 h using 1,2-dichloroethane (DCE) as a solvent.
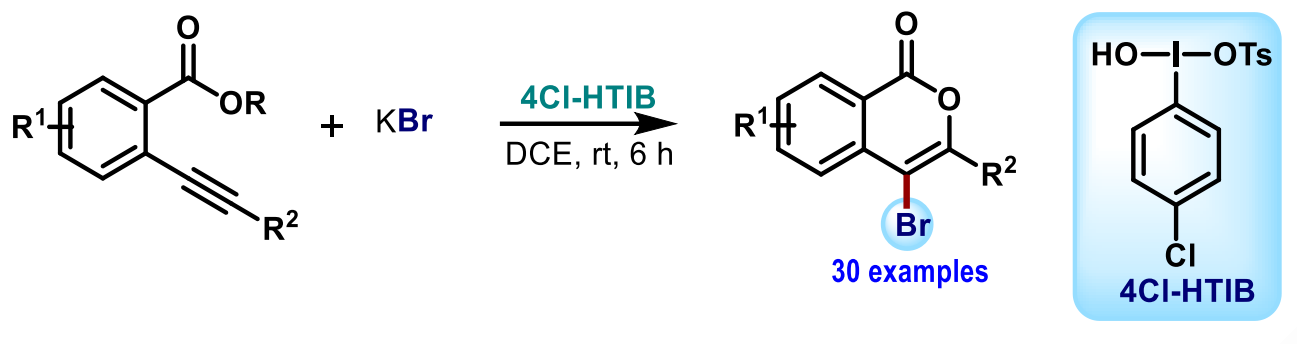
Using this method, a broad range of halogenated isocoumarins could be obtained in mostly good yields. The reaction involves a bromoiodane that was generated in situ from the hypervalent iodine reagent and KBr. The protocol was scaled up to the 2 mmol scale, giving a yield of 88 % in a model reaction. The 4-bromoisocoumarin products can be further transformed, e.g., via a reductive debromination, a Miyaura coupling, or a palladium-catalyzed alkyne annulation.
- Metal‐free Synthesis of 4‐Bromoisocoumarins through Brominative Annulation of 2‐Alkynylaryloate Esters using in-situ-generated transient Bromoiodane,
Mohammad Sodoor, Sermadurai Selvakumar,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300925