CO2 to C2H2
Use of the greenhouse gas CO2 as a chemical raw material would not only reduce emissions, but also the consumption of fossil feedstocks. A novel metal-free organic framework could make it possible to electrocatalytically produce ethylene, a primary chemical raw material, from CO2. As a team around Chengtao Gong and Fu-Sheng Ke from Wuhan University, China, has reported, nitrogen atoms with a particular electron configuration play a critical role for the catalyst.
Ethylene is an essential starting material for many products, including polyethylene and other plastics. Ethylene is produced industrially by the high-energy cracking and rectification of fossil feedstocks. The electrochemical conversion of CO2 to ethylene would be a promising route to reducing CO2 emissions while also saving energy and fossil resources.
Building A Catalyst
CO2 is very stable, which makes it difficult to induce into reaction. With the use of electricity and catalysts, it is currently possible to convert it into C1 chemicals such as methanol and methane. The additional challenge in producing ethylene is that a bond must be formed between two carbon atoms. This has previously only been achieved with copper catalysts. Metal-free electrocatalysis would be advantageous because metals are a cost factor and can cause environmental problems.
The researchers have developed a metal-free electrocatalyst for the conversion of CO2 to ethylene. The catalyst is based on a nitrogen-containing covalent organic framework (COF). COFs are a class of porous, crystalline, purely organic materials with defined topology. In contrast to metal-organic frameworks (MOFs), they require no metal ions to hold them together. Their pore sizes and chemical properties can be tuned over a wide range through selection of the building blocks.
Proper Electron Configuration
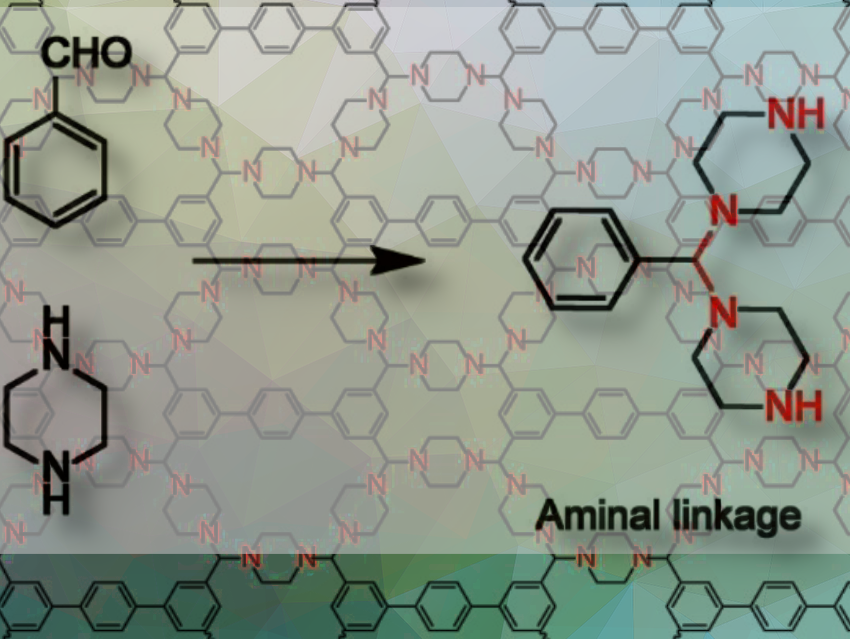
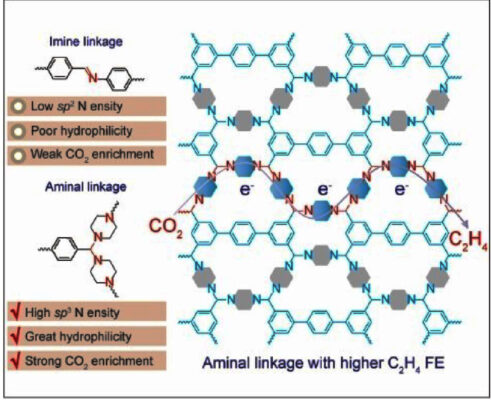
The new COF contains nitrogen atoms with sp3 hybridization as catalytically active centers. These sp3 nitrogen centers bind the individual building blocks into a framework through an aminal link (two amino groups bound to one carbon atom; pictured above).
 In contrast to COFs with a classic imine-linkage (–C=N–), aminal COFs have strict requirements regarding the lengths and angles of the bonds between building blocks, which causes the frameworks to be formed through ring closures. The researchers found a suitable combination by using piperazine and a building block made of three aromatic, six-membered carbon rings.
In contrast to COFs with a classic imine-linkage (–C=N–), aminal COFs have strict requirements regarding the lengths and angles of the bonds between building blocks, which causes the frameworks to be formed through ring closures. The researchers found a suitable combination by using piperazine and a building block made of three aromatic, six-membered carbon rings.
When used as electrodes, their new COFs demonstrated high selectivity and performance (Faraday efficiency up to 19.1%) for the production of ethylene. Success of the aminal COFs is due to the high density of active sp3-nitrogen centers, which both very effectively capture CO2 and transfer electrons. This results in a high concentration of excited intermediates that can undergo C–C coupling. In contrast, a variety of imine-linked COFs, which contain sp2 nitrogen instead of sp3, were similarly tested and produced no ethylene. This proves the importance of proper electron configuration for the electrochemical reduction of CO2 to ethylene.
- Linkage Engineering in Covalent Organic Frameworks for Metal-Free Electrocatalytic C2H4 Production from CO2,
Yang Xiao, Jie Lu, Kean Chen, Yuliang Cao, Chengtao Gong, Fu-Sheng Ke,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202404738