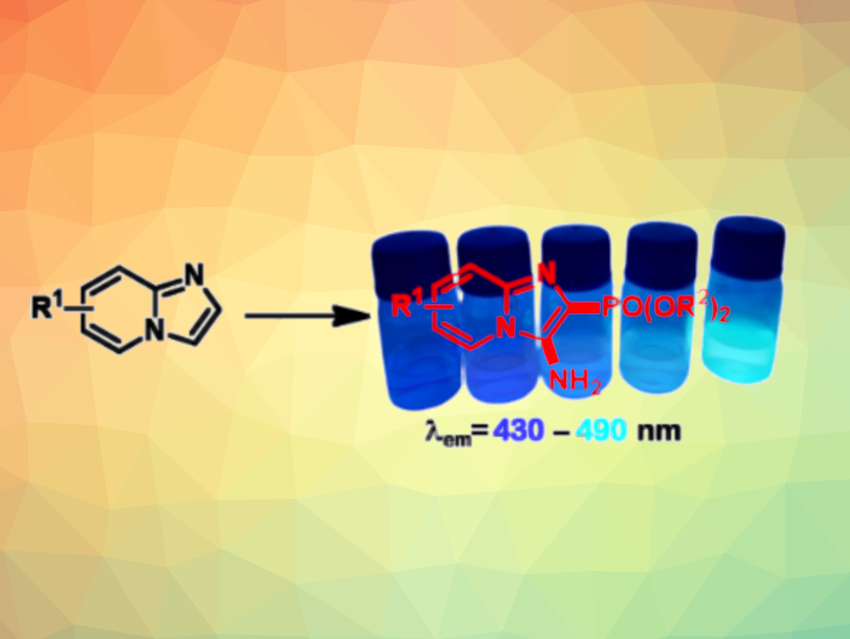
Phosphonated aromatic compounds constitute a class of organophosphorus compounds with potential applications in, e.g., catalysis, pharmaceutical chemistry, agrochemistry, and materials science. For example, they can serve as fluorescent probes. Phosphonated imidazopyridines are interesting in this context.
Aurelio G. Csáky, Universidad Complutense, Madrid, Spain, and colleagues have developed a metal-free procedure for the direct aminophosphonation of imidazo[1,2-a]pyridines in green solvents under air (general reaction pictured). The team reacted a variety of imidazo[1,2-a]pyridines with tBuONO in acetone to introduce a nitroso (NO) substituent, followed by a reaction with a phosphonation reagents of the type P(OR)3 using tris(pentafluorophenyl)borane (BCF) as a catalyst. The method is a one-pot process, avoiding the isolation of unstable nitrosoarene intermediates.
Under these conditions, the desired 3-(aminoimidazo[1,2-a]pyridin-2-yl)phosphonates were obtained in moderate to good yields. The protocol shows a good functional group tolerance, scalability, and does not require anhydrous solvents or an inert atmosphere. The products absorb light in the UV–Vis region and emit blue to greenish fluorescence (examples pictured). They could be interesting for applications as fluorescent probes.
- Metal‐Free Aminophosphonation: Eco‐friendly Synthesis and Photophysical Properties of Fluorescent 3‐(Aminoimidazo[1,2‐a]pyridin‐2‐yl)phosphonates,
Aurelio G. Csaky, Silvia Roscales,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202412300