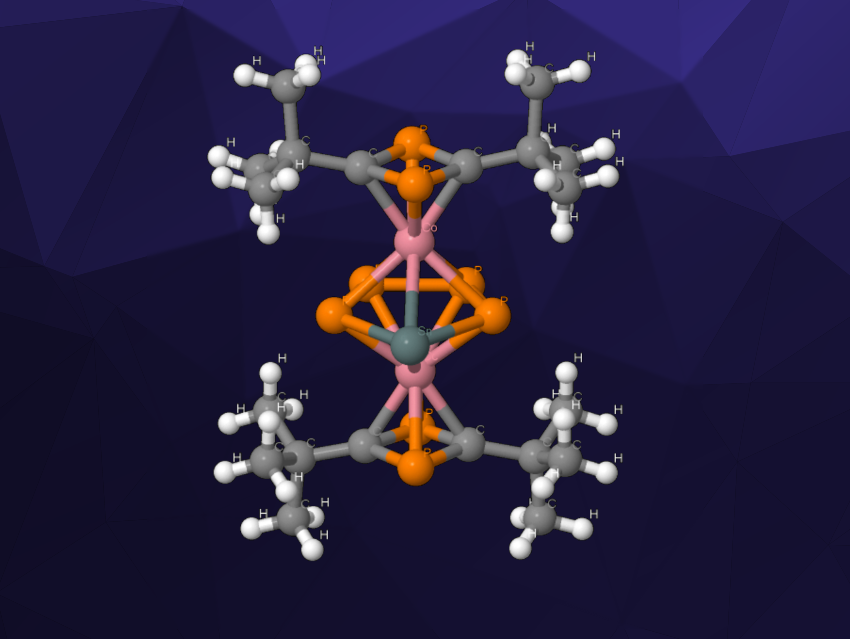
Cyclopentadienyl (Cp) ligands are widely used in organometallic chemistry. Phosphorus analogues of cyclopentadienyl are also useful ligands. Derivatives that incorporate both phosphorus and heavy group 14 elements (i.e., Si, Ge, Sn, or Pb), however, are not well-studied.
Robert Wolf, University of Regensburg, Germany, and colleagues have synthesized the first metal complexes with the heavy cyclopentadienyl analogue ligands SnP42– and PbP42–. The complexes are triple-decker sandwich complexes of the type [(η4–tBu2C2P2)2Co2(μ,η5:η5–P4E)] (E = Sn (pictured), Pb). The team’s strategy involves the reaction of white phosphorus (P4) with tetrel cobaltate complexes, i.e., [2,6-Dipp2-C6H3–ECo(η4-P2C2tBu2)(η4-COD)] (Dipp = 2,6-iPr2–C6H3, COD = 1,5-cyclooctadiene) or Sn[Co(η4-P2C2tBu2)(COD)]2.
These reactions gave the SnP42– complex and the analogous lead complex. The latter, however, is unstable at room temperature and decomposes under loss of the lead atom. The structures of the products were confirmed using X-ray crystallography. The team used quantum chemical calculations to investigate the aromaticity of the EP42– ligands and found evidence of a substantial aromatic character. According to researchers, the ligands might be useful in the synthesis of further unusual compounds.
- Transition-Metal-Stabilized Heavy Tetraphospholide Anions,
John A. Kelly, Verena Streitferdt, Maria Dimitrova, Franz F. Westermair, Ruth M. Gschwind, Raphael J. F. Berger, Robert Wolf,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08754