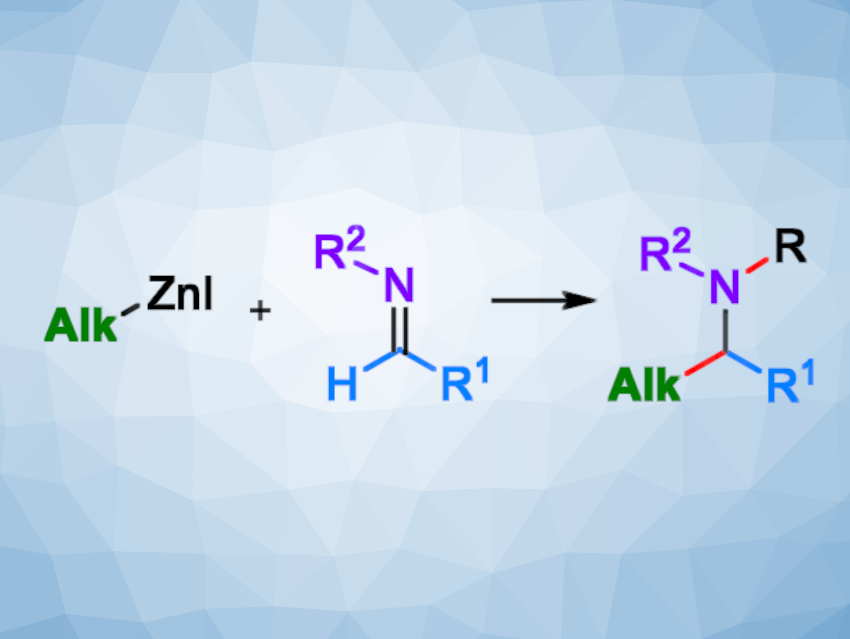
The addition of an organometallic species to an imine, known as a Mannich-type reaction, is a powerful method for the preparation of nitrogen-containing products. However, in the case of zinc-based reagents, these reactions have often been limited to the use of symmetric diorganozinc species, such as commercially available diethylzinc. The use of more atom-economic mixed organozinc species is far less common.
Marc Presset, Erwan Le Gall, Institut de Chimie et des Matériaux Paris-Est, Thiais, France, and colleagues have developed different strategies for the use of alkylzinc halides in Mannich-type reactions (pictured). The team prepared different reagents by metalation of the corresponding halides with Zn dust in THF or CH3CN, in some cases in the presence of a stoichiometric amount of LiCl. Then they studied their reactivity towards a 2-thiophenesulfonyl imine as a substrate. i-PrZnI gave the highest yield and was used as a model reagent for further reactions.
The team found that reactions with sulfonyl imines occurred upon simple heating to give the desired products in moderate to excellent yields (14 examples, 30–99 % yield), while the use of N-alkyl imines required an additional activation by acetyl chloride (12 examples, 49–86 %) or by trimethylsilyl chloride (14 examples, 26–87 %).
These methods are complementary, leading to the preparation of either N-protected secondary or tertiary amines or N-unprotected secondary amines in good yields. Due to their atom economy, alkylzinc reagents can, thus, be considered a useful alternative to the more commonly used diorganozinc compounds.
- Mannich‐Type Reactions of Alkylzinc Reagents,
Marine Pinaud, Emilie Plantiveau, Eric Huet, Erwan Le Gall, Marc Presset,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300572