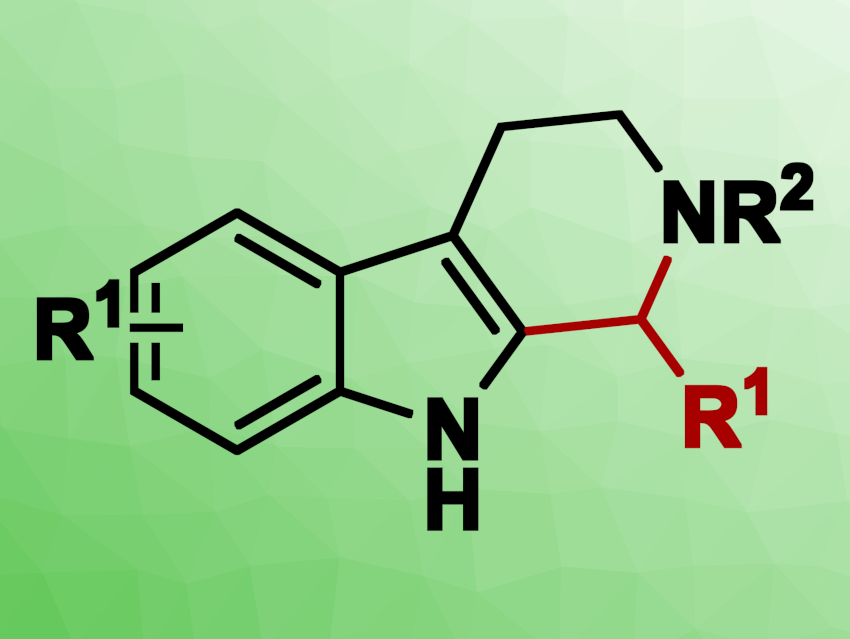
Tetrahydro-β-carboline (THBC) derivatives are frequently used as drugs or drug candidates. One commonly used method for constructing compounds with a THBC core is the Pictet–Spengler reaction, wherein tryptamine and aldehydes react in the presence of Lewis acids or Brønsted acids. However, aldehydes can be difficult to store and handle and can be prone to side reactions. Thus, a transition-metal-catalyzed dehydrogenation approach can be beneficial for the synthesis of THBCs. In this reaction sequence, a catalytic dehydrogenation of an alcohol is used to generate a reactive aldehyde or ketone intermediate in-situ.
Zhihui Shao, Henan Agricultural University, Zhengzhou, China, and colleagues have developed an efficient and green method for the synthesis of tetrahydro-β-carbolines via the dehydrogenative coupling of alcohols with tryptamine (general reaction pictured below). The team used an Mn PNP pincer catalyst (pictured below) in the presence of Na2CO3 as a weak base. The reactions were performed in dioxane at 165 °C.
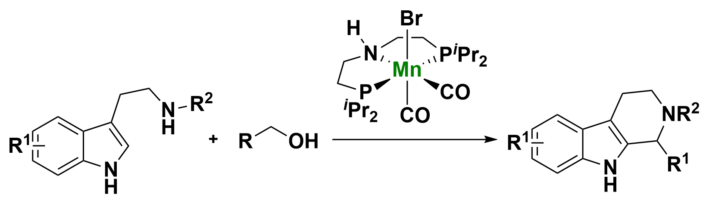
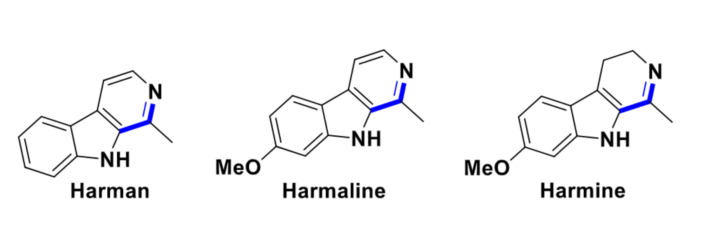
Using this approach, a wide range of THBCs (> 40 examples) was obtained with high selectivity (up to 99 % yield). A broad range of benzylic and aliphatic alcohols with different functional groups were suitable substrates for this reaction. The developed catalytic system, in combination with an oxidation step, also enabled the synthesis of the pharmaceutically relevant molecules harman, harmaline, and harmine in a concise manner.
- Synthesis of Tetrahydro‐β‐carbolines via Manganese‐Catalysed Oxidative Pictet−Spengler Reaction,
Zhihui Shao, Xiaoyu Zhang, Xinyan Li, Weilin Wang, Jiaqiao Ding, Bing Cui, Mingqin Zhao,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202203758