Martin Kotora, from Charles University in Prague, Czech Republic, discusses recent work on blue-light-induced photorearrangement of quinones, a study he recently published together with his former Ph.D. student Alexander A. Fadeev in ChemistryEurope. The focus of this work on natural products makes it more than just a synthetic protocol.
Can you describe the methodology and key findings of your recent study on the photochemistry of quinones?
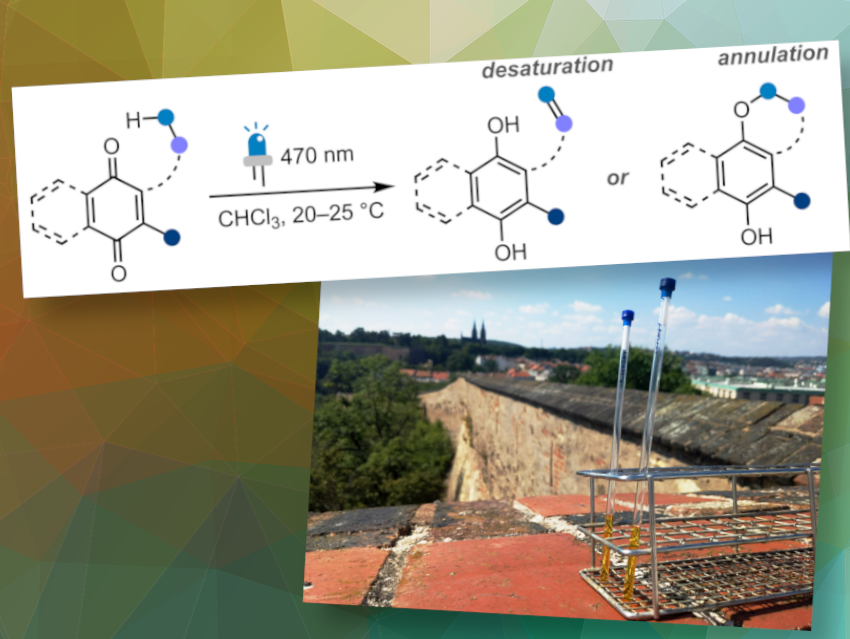
We performed a systematic study to explore the behavior of quinones under blue light irradiation in solution.
First, we obtained a series of representative quinones of different classes and irradiated them under the benchmark reaction conditions. Thus, we were able to engage alkyl, allyl, alkenyl, and aryl quinones to react preferentially in an intramolecular photoredox fashion, allowing us to prepare ten natural phenolic compounds.
Next, we demonstrated how the reaction course can be changed in a different reaction medium, using thymoquinone as an example. With these findings, and in conjunction with existing literature data, we summarized and classified the possible mechanistic pathways of the presented transformations, aiming to make the photochemistry of quinones more predictable and user-friendly.
Why are you interested in this?
It has long been known that quinones are light-sensitive. However, it is not always clear what happens to them under light and what the end products of the light-induced processes are. Considering the widespread occurrence of quinones in nature and their extensive use as organic redox-active materials, it is important to answer these questions.
Although many studies have been devoted to this topic previously, we have found several gaps in knowledge. For example, the first experimental confirmation of the involvement of spirocyclopropanes in the photochemistry of quinones was obtained only recently in our laboratory [1].
In this work, we aimed to disclose a general protocol for the photorearrangement of quinones into phenolic compounds, which could be used to predict the presence of these substances in natural sources.
What is new and cool about it?
Natural product anticipation is a novel direction in organic synthesis, aiming to predict and access natural compounds before their isolation from a natural source is reported.
By examining the photochemistry of quinones from this perspective, and using the examles of their co-occurrence with natural phenolics, we demonstrated how these molecules can be anticipated in natural sources based on each other’s presence.
Ultimately, the work’s focus on natural products makes it more than just a synthetic protocol.
What is the main significance of your results?
The results indicate that monochromatic blue light effectively induces the intramolecular photorearrangement of a broad variety of substituted benzo- and naphthoquinones, despite strong deviations in their absorption profiles. The general nature of this transformation allows for the preparation of natural and unnatural phenolic compounds of different classes, such as hydroquinones, chromenes, 2,3-dihydrobenzofurans, and benzofurans.
Among the ten natural phenolic compounds prepared by this method, five were earlier found in nature together with the quinones used as their precursors. These cases exemplify how natural phenolics can be anticipated through the photochemistry of quinones.
Besides that, this work illustrates that the redox cycling between quinones and hydroquinones can also involve the triplet quinone biradicals in addition to semiquinone radical anions.
What is the longer-term vision for your research?
We believe that our findings will help to explain the origin of certain natural quinones and phenols and to develop new synthetic approaches to these compounds. However, the photoreactivity of quinones may not only be useful for the anticipation and synthesis of natural products, but also for determining the degradation pathways of artificial compounds.
For instance, tert-butylbenzoquinone (TBBQ) was previously found in food due to the oxidation of tert-butylhydroquinone (TBHQ), which is used as a preservative. We showed that TBBQ is readily converted to methallyl hydroquinone under visible light at room temperature. Hence, the presence of methallyl hydroquinone can be expected in foods containing TBHQ, if this conversion occurs under the storage conditions. However, the properties of methallyl hydroquinone have not been well-studied yet, in contrast to the properties of its structural relative TBHQ.
In this respect, the photorearrangement of quinones into phenolic compounds provides attractive targets for further studies.
What part of your work was the most challenging?
In the beginning, it was hard to foresee or explain the fate of any particular quinone under irradiation based solely on the literature precedents, because the majority of the earlier work was done with different irradiation sources or using different solvents. Still, we tried to compare our results with the available literature data where possible.
Fortunately, the challenge was getting smaller with the growing number of cases developed in our laboratory.
Anything else you would like to add?
I would like to emphasize that this presented work was done single-handedly by Alexander. He is the one who deserves credit for his diligent and persevering work.
Thank you very much for sharing these insights.

Martin Kotora (left) is a Professor at the Department of Organic Chemistry in the Faculty of Science at the Charles University in Prague, Czech Republic. Alexander Fadeev (right) is currently a researcher at the same institute.
The paper they talked about:
- Anticipating Natural Phenolics Through Visible Light-Induced Photorearrangement of Quinones,
Alexander A. Fadeev, Martin Kotora,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202400094
Reference
[1] Alexander A. Fadeev, Daniel Bím, Ivana Císařová, Martin Kotora, Bioinspired intramolecular spirocyclopropanation of quinones as an interrupted photoredox process, Org. Chem. Front. 2024, 11, 5703. https://doi.org/10.1039/D4QO01291G





To Professors Kotora and Fadeev: Your research on the photochemistry of quinones is very interesting. Congratulations on your fascinating results. I studied the photochemistry of alpha, beta-unsaturated enones when I was a graduate school. It is well known that these enones undergo photo-reduction in the presence of hydrogen donors. I wonder if you always have looked into this possibility since in natural products, there is no lack of those species that could influence the photochemical reaction outcome of your TBHQ. Just a thought!