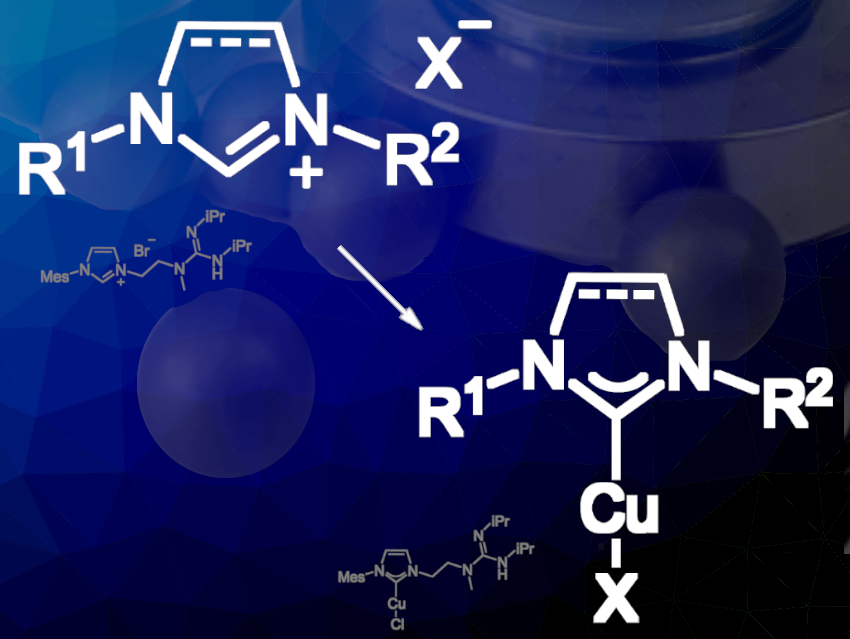
Green chemistry aims to minimize or completely prevent chemical waste by using harmless chemicals, replacing hazardous reagents, and implementing safer chemical processes. Copper(I)/N-heterocyclic carbene complexes are versatile catalysts commonly synthesized by preparing an intermediate silver(I)/NHC complex followed by transmetallation to copper(I). However, this approach produces significant amounts of transition metal waste and uses solvents, which goes against green chemistry principles.
Johannes F. Teichert, Technische Universität Chemnitz, Germany, and colleagues have developed a one-pot two-step procedure that eliminates the use of silver(I) complexes, reduces waste formation, and minimizes solvent usage. The team employed mechanochemical synthesis using a planetary ball mill in an argon-filled glovebox, where they mixed and ground copper(I) chloride, an imidazolium salt, and K3PO4 as a base for four hours. After purification steps, they obtained a bifunctional catalyst that effectively catalyzed various reduction/hydrogenation transformations using dihydrogen as a terminal reducing agent.
The team also demonstrated that their protocol is applicable to the synthesis of other standard copper(I)/NHC complexes, proving that transition metal complexes with functional groups can be prepared through ball milling synthesis. This study highlights the potential of this approach for the atom-economic preparation of other complex catalysts, which are difficult or wasteful to be prepared by typical liquid state synthesis methods.
- Mechanochemical solid state synthesis of copper(I)/NHC complexes with K3PO4,
Ina Remy-Speckmann, Birte M. Zimmermann, Mahadeb Gorai, Martin Lerch, Johannes F. Teichert,
Beilstein J. Org. Chem. 2023, 19, 440–447.
https://doi.org/10.3762/bjoc.19.34