Molecules that reversibly change their color and/or luminescent behavior in response to mechanical stimuli are interesting research targets with different potential applications. For example, mechanochromic molecules can be useful for mechanical sensors, the detection of defects and deformations, and secure printing. However, there are only a few examples of mechanochromic organic molecules that exhibit luminescence in the red to near-infrared (NIR) region.
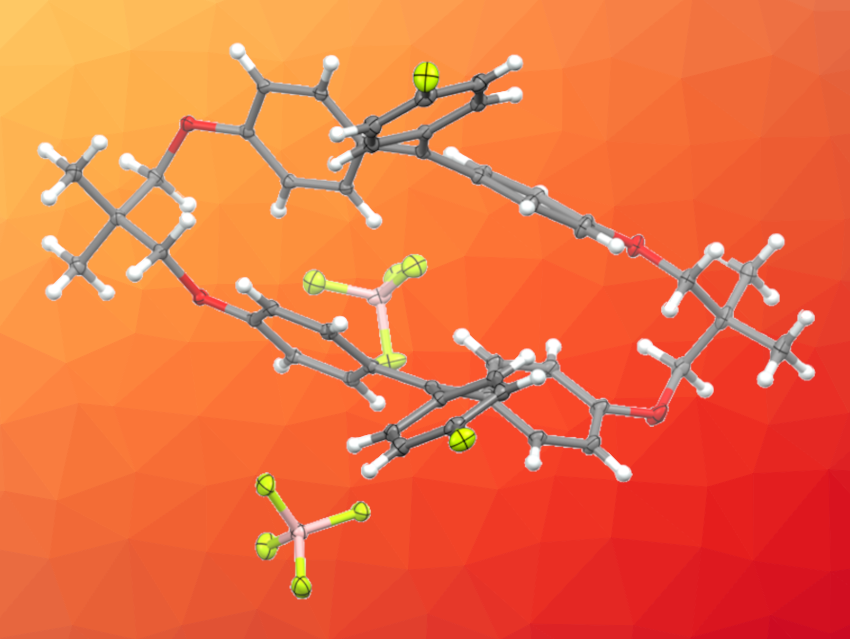
Yusuke Ishigaki, Hokkaido University, Japan, and colleagues have designed macrocyclic dications with two triarylmethylium units (pictured below). The team synthesized the dications starting from 4,4′-dihydroxybenzophenone, which was reacted with tosylated 2,2-dimethyl-1,3-propanediol in a stepwise manner to obtain a macrocyclic diketone as a key intermediate. The diketone was reacted with aryl lithium reagents, converted into a dimethyl ether, and transformed into the desired dication under acidic conditions.
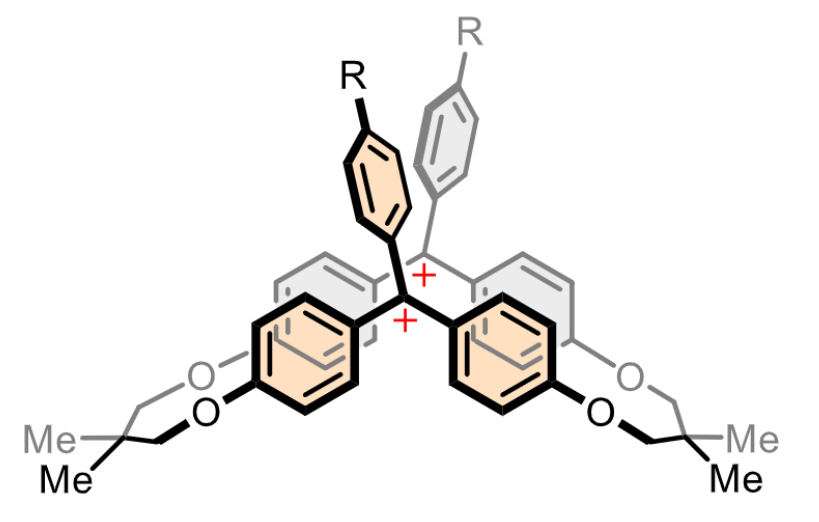
The researchers found that the macrocyclic dications exhibit mechanochromic luminescence in the red to NIR region upon grinding. According to the team, this is the first example of mechanochromic luminescence based on carbocations. The work may suggest a design strategy for creating different stimuli-responsive materials.
- Bis(triarylmethylium)‐type Macrocyclic Dications: Mechanochromic Emission Extending to the Red Region,
Yusuke Ishigaki, Takuya Tachibana, Kazuma Sugawara, Moto Kikuchi, Takanori Suzuki,
ChemPlusChem 2023.
https://doi.org/10.1002/cplu.202300110