A lithium ceramic could act as a solid electrolyte in a more powerful and cost-efficient generation of rechargeable lithium-ion batteries. The challenge is to find a production method that works without sintering at high temperatures. Jennifer L. M. Rupp, Massachusetts Institute of Technology (MIT), Cambridge, USA, and Technical University Munich, Garching, Germany, and colleagues have developed a “sinter-free” method for the efficient, low-temperature synthesis of these ceramics in a conductive crystalline form.
Solid-State Batteries for Electric Vehicles
Two factors dominate the development of batteries for electric vehicles: power, which determines the vehicle range, and cost, which is critical in the competition with internal combustion engines. The US Department of Energy aims to accelerate the transition from gasoline-powered vehicles to electric vehicles and has set ambitious goals for reducing production costs and increasing the energy density of batteries by 2030. These targets cannot be achieved with conventional lithium-ion batteries.
A promising approach to making smaller, lighter, significantly more powerful, and safer batteries is to use solid-state cells with anodes made of metallic lithium instead of graphite. In contrast to conventional lithium-ion batteries, which have liquid organic electrolytes and use a polymer film to separate the anodic and cathodic compartments, all components of a solid-state battery are solids. A thin ceramic layer simultaneously functions as a solid electrolyte and separator. It is very effective against both the dangerous short circuits caused by the growth of lithium dendrites and thermal runaway. In addition, the batteries contain no easily inflammable liquids.
Lithium Garnet as a Solid-State Electrolyte
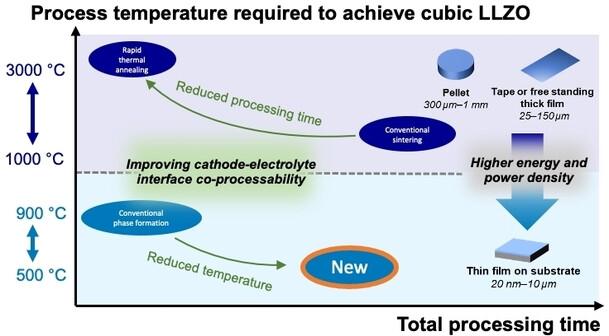
A suitable ceramic electrolyte/separator for cells with high energy density is the garnet-type lithium lanthanum zirconium oxide Li7La3Zr2O12−d (LLZO). This material must usually be sintered together with the cathode at over 1050 °C to convert the LLZO to the rapid lithium-conducting cubic crystalline phase, sufficiently densify it, and strongly bind it to the electrode.
However, temperatures above 600 °C destabilize sustainable low-cobalt or cobalt-free cathode materials, while also driving up production costs and energy consumption. New production methods that are more economical and sustainable are needed. The team has developed such a new synthetic process.
New Low-Temperature Processing Route
The new process is not based on a ceramic precursor compound, but a liquid one, which is directly densified to form LLZO in a sequential decomposition synthesis (SDS). The precursor solution for SDS was prepared by dissolving LiNO3, Al(NO3)3·9H2O, La(NO3)3·6H2O, and zirconium(IV) acetylacetonate in a solvent mixture consisting of methanol, 1-methoxy-2-propanol, and bis(2-ethylhexyl) phthalate. MgO was used as the substrate for the SDS deposition. To deposit a film, the SDS precursor solution was pumped through a polypropylene syringe into a spray atomizer and deposited onto the MgO substrate, which had been placed on a heated stainless-steel hot plate.
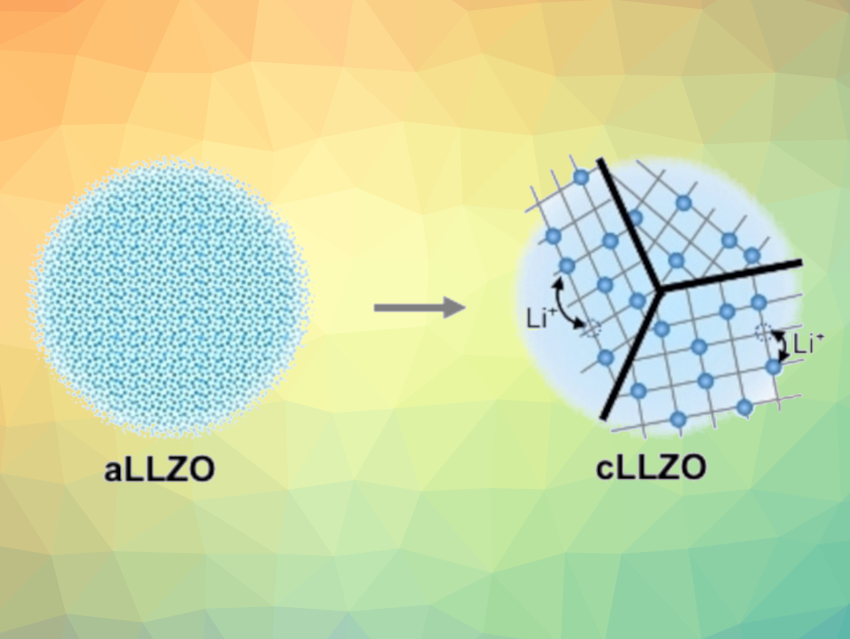
To optimize the conditions for the synthesis, the team analyzed the multistep phase transformation of LLZO from the amorphous form (aLLZO) to the required crystalline form (cLLZO) using a variety of methods (Raman spectroscopy, dynamic differential scanning calorimetry) and produced a time-temperature-transformation diagram.
Based on the insights they gained into the crystallization process, the team developed a route by which cLLZO is obtained as a dense, solid film after 10 h of annealing at the relatively low temperature of 500 °C. For future battery designs, this method could allow for the integration of the solid LLZO electrolyte with sustainable cathodes that could avoid the use of socioeconomically critical elements such as cobalt.
- Time‐Temperature‐Transformation (TTT) Diagram of Battery‐Grade Li‐Garnet Electrolytes for Low‐Temperature Sustainable Synthesis,
Yuntong Zhu, Michael Chon, Carl V. Thompson, Jennifer L. M. Rupp,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202304581