Lignin, a complex aromatic polymer found in wood and other lignocellulosic biomass, could be a useful feedstock for the production of bio-based aromatic chemicals. However, there is a lack of efficient conversion processes for lignin. Pyrolysis is a promising approach, but the re-condensation of intermediates is a problem. Ether bonds in lignin are easily cleaved at approximately 350 °C. However, the yield of depolymerization products from lignin pyrolysis is generally much lower than expected. This can be explained by re-condensation of the pyrolysis products.
Haruo Kawamoto, Kyoto University, Japan, and colleagues have found that the use of diphenoxybenzene as an aprotic solvent together with 1,2,3,10b-tetrahydrofluoranthene as a hydrogen donor can effectively suppress the re-condensation during lignin pyrolysis, giving oligomers in high yields of over 80 %. The team studied the pyrolysis of Japanese cedar wood at 270–380 °C. Extraction with ethyl acetate and water was used to separate the lignin-derived oligomers from polysaccharide-derived products.
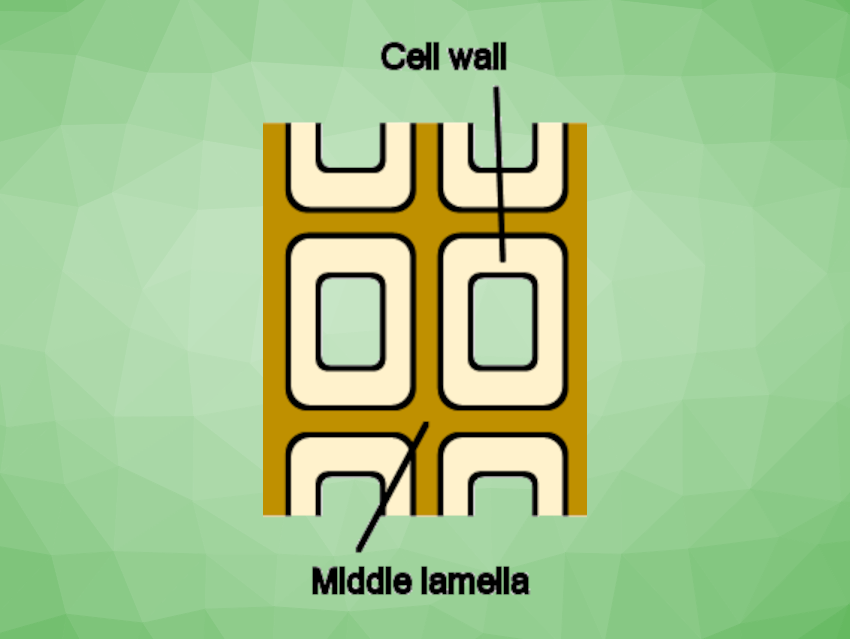
The team found that the oligomer formation is highly dependent on the pyrolysis temperature: The yield of lignin-derived oligomer was as low as ca. 20 % at 270 °C. According to the team, a possible explanation is that these lignin-derived products come from the middle lamella connecting the cells in the wood (pictured). The yield significantly increased to ca. 80 % when the cellulose in the wood cell walls was thermally degraded by increasing the temperature above 300 °C.
- Stable oligomer formation from lignin by pyrolysis of softwood in an aprotic solvent with a hydrogen donor,
J. Q. Wang, E. Minami, H. Kawamoto,
ChemistryOpen 2022.
https://doi.org/10.1002/open.202200104

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

