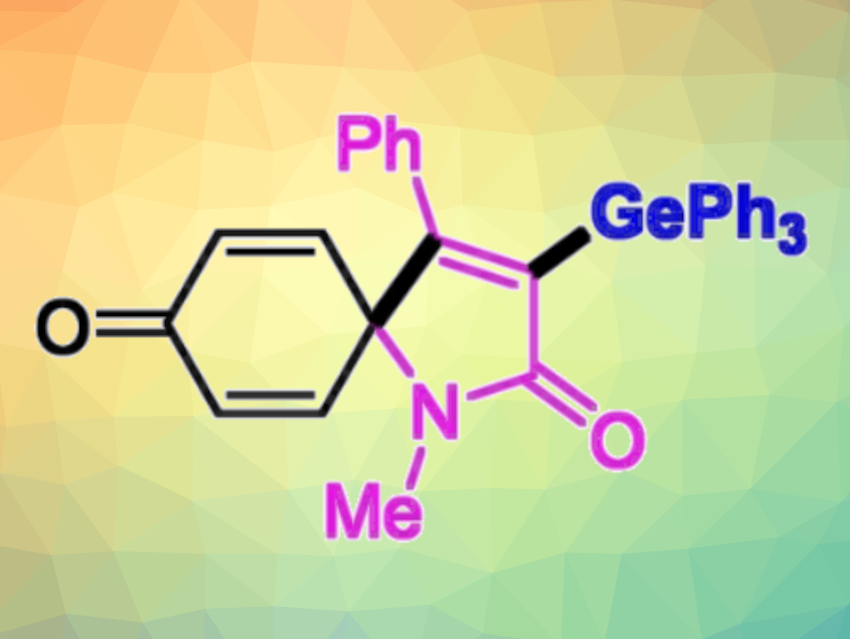
Organogermanium compounds can have interesting physical, chemical, and biological properties. They can also be useful intermediates in organic synthesis. Therefore, methods for the selective synthesis of organogermanium compounds are interesting research targets.
Leiyang Lv, Zhiping Li, Renmin University of China, Beijing, and colleagues have developed a method for the light-promoted regioselective germylation of N-arylpropiolamides or aryl alkynoates with germanium hydrides at room temperature in the absence of a transition-metal catalyst (pictured below). The team reacted N-arylpropiolamides with germanium hydrides of the type R3GeH, using Eosin Y as a photocatalyst, tert-butyl hydroperoxide (TBHP) as an oxidant, and a t-BuOH/H2O mixture as the solvent under blue LED light at 25 °C. For aryl alkynoate substrates, they used di-tert-butyl peroxide (DTBP) as an oxidant and PhCl as the solvent and performed the reactions under a violet LED light at 25 °C.
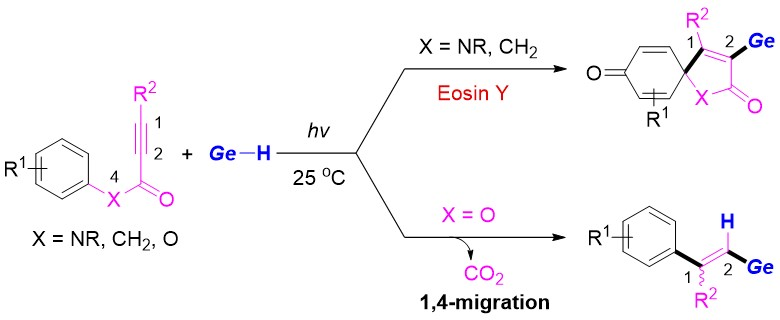
The team found the spirocyclization is the key step in these transformations, and different products are then formed depending on the linker of the substrates. In the case of N-arylpropiolamides, germyl-containing spiro[4.5]trienones were obtained selectively via sequential C−Ge and C−C bond formations, while aryl alkynoates gave vinylgermanes via a radical Smiles rearrangement and CO2 release.
The desired products were obtained in moderate to good yields, and a variety of functional groups were tolerated. This protocol provides an atom-economic and operationally simple path to organogermanium products.
- Light‐Promoted Germylation of Aryl Propiolamides/Alkynoates: Synthesis of Ge‐Containing Spiro[4.5]trienones and Vinylgermanes,
Yani Luo, Leiyang Lv, Zhiping Li,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300467