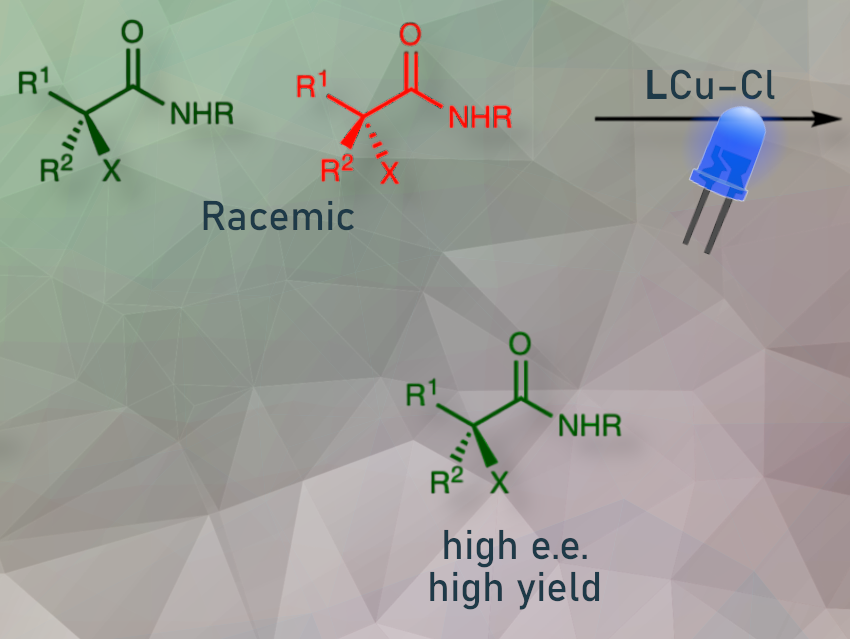
Deracemization is a promising method for producing enantiomerically enriched compounds by converting both enantiomers of a racemic starting material into a single enantiomer, often using a light-activated catalyst. While there is strong proof of concept, significant challenges remain. In particular, breaking carbon-heteroatom bonds, not just C–H or C–C bonds, and producing broad classes of useful enantiomerically enriched compounds and tetrasubstituted stereocentres.
Peng Liu, University of Pittsburgh, PA, USA, Gregory C. Fu, California Institute of Technology (CalTech), Pasadena, CA, USA, and colleagues use a chiral copper catalyst prepared in situ from commercially available components (CuX and (R)- or (S)-DTBM-SEGPHOS) to achieve photoinduced deracemization of tertiary (and secondary) alkyl halides via carbon-halogen bond cleavage.
The catalyst contains a copper chloride center attached to a bulky chiral phosphine ligand. Upon light activation, it undergoes a single electron transfer with an alkyl halide, breaking the alkyl halide’s carbon-halide bond and converting it to a radical. This is followed by chloride transfer from the catalyst. The bulky chiral phosphine ligand directs it toward one enantiomer.
The researchers found that the method has worked well over a range of alkyl halides, giving high yields and enantioselectivity.
- Photoinduced copper-catalysed deracemization of alkyl halides,
Feng Zhong, Renhe Li, Binh Khanh Mai, Peng Liu, Gregory C. Fu,
Nature 2025, 640, 107–113.
https://doi.org/10.1038/s41586-025-08784-8