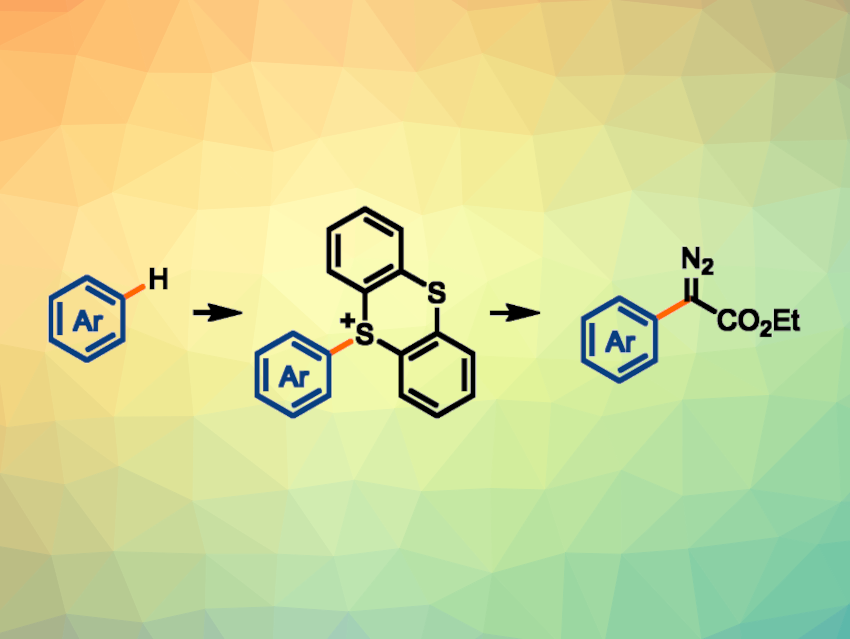
Arenes functionalized with diazoalkane groups can be useful intermediates in organic synthesis. However, the synthesis of complex aryl diazo compounds is generally challenging due to the high reactivity of diazoalkanes and the facile decomposition of the products.
Tobias Ritter, May Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a method for the synthesis of aryl diazoacetates via arylthianthrenium salts (general reaction pictured). The team used a two-step process, based on a site-selective C–H thianthrenation of the arene, which is followed by the oxidative addition of the resulting arylthianthrenium salts to Pd(0) and a reaction with ethyl diazoacetate. The Pd-catalyzed reactions were performed using Pd(PPh3)4 in the presence K2CO3 as a base.
Using this approach, the researchers achieved the introduction of a diazo group into densely functionalized arenes at a late stage. The resulting products can be used in a variety of further transformations, such as cyclopropanations, insertions into O–H or N–H bonds, or aziridinations.
- Late‐Stage Diazoester Installation via Arylthianthrenium Salts,
Le Li, Sven Müller, Roland Petzold, Tobias Ritter,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202419931