Cyclophanes such as [2.2]paracyclophane derivatives (pictured) can show planar chirality. Such compounds could be useful, e.g., in asymmetric catalysis or polymer chemistry. However, the asymmetric synthesis of planar-chiral [2.2]paracyclophanes can be challenging. The kinetic resolution of racemic samples could be an alternative path to enantiopure paracyclophane derivatives.
Kazuaki Kudo, The University of Tokyo, Japan, and colleagues have developed a resin-supported peptide catalyst for the kinetic resolution of a planar-chiral [2.2]paracyclophane derivative. The team’s approach is based on the Michael addition of a malonate ester to a nitroalkene substituent on the paracyclophane structure (pictured below). The resin-supported catalyst consists of a helical peptide with a Trp(N-Me)-Trp-(Leu-Leu-Aib)3 sequence whose N-terminus was functionalized with a strongly basic guanidinyl group. The catalytic activity stems from the guanidinyl group, while the helical peptide provides the necessary chiral environment.
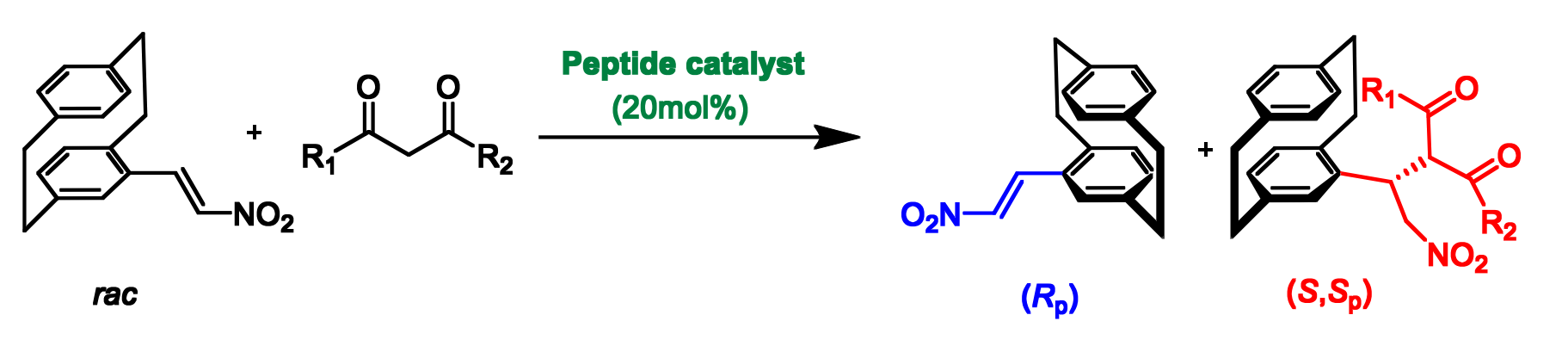
The reaction was performed in toluene at –10 °C and gave the (S,Sp)-adduct (pictured above in red) with high diastereo- and enantioselectivity. The (Rp)-isomer of the [2.2]paracyclophane (pictured above in blue) was successfully recovered. Overall, the work provides a new pathway to enantiopure paracyclophane derivatives characterized by both planar and central chirality.
- Kinetic Resolution of a Planar‐Chiral [2.2]Paracyclophane via Michael Addition to Nitroolefins Catalyzed by N‐Terminal Guanidinylated Helical Peptide,
Jiaqi Tian, Kenya Tamaribuchi, Isao Yoshikawa, Kazuaki Kudo,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400117
![Kinetic Resolution of a Planar-Chiral [2.2]Paracyclophane](https://www.chemistryviews.org/wp-content/uploads/2024/03/kineticresolutionparacyclophane_2024.png)




I want to learn how these molecules are made.