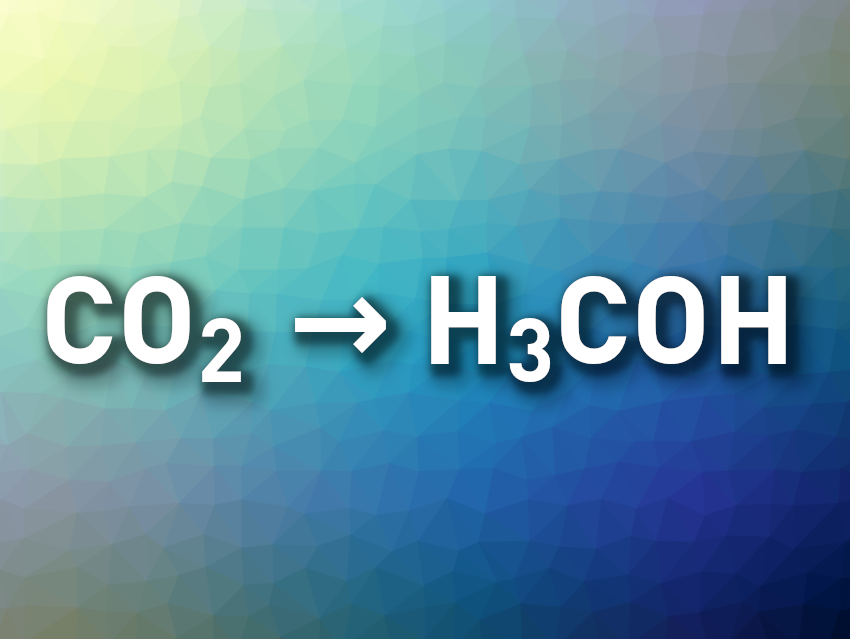Using the greenhouse gas CO2 as a chemical feedstock is interesting from a sustainability perspective. The electrochemical reduction of carbon dioxide to fuels such as formic acid or methanol could, for example, be used to store energy from renewable sources while reducing the amount of CO2 released into the atmosphere. Methanol, in particular, can serve as an easy-to-store fuel and also as a C1 building block in chemical syntheses. However, suitable molecular electrocatalysts for the selective formation of methanol from CO2 are rare.
Abhishek Dey, Indian Association for the Cultivation of Science, Kolkata, India, and colleagues have found that an iron chlorin (a partially hydrogenated porphyrin derivative) can be used to electrochemically reduce CO2 to either formic acid/formate or methanol depending upon the reaction conditions. The team used an iron chlorin complex called FeTEsC, which has four electron-withdrawing –CO2Et groups and an amine substituent.
This selectivity of the reduction depends on the acidity/water concentration of the acetonitrile/tetrahydrofuran (MeCN/THF) electrolyte. Using the same catalyst, CO2 can be reduced to either formate or to methanol. Methanol is formed in a Faradaic yield of up to 48 % at a water concentration of 2 M. According to the researchers, hydrogen bonding from the pendent amine likely allows the 6e–/6H+ reduction of CO2 to methanol by stabilizing key Fe(I)-based intermediates. Under less acidic conditions with a lower water concentration, formate is selectively formed in over 90 % Faradaic yield.
- Electrochemical Reduction of CO2 to CH3OH Catalyzed by an Iron Porphyrinoid,
Paramita Saha, Sk Amanullah, Sudip Barman, Abhishek Dey,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.4c08922