Metathesis reactions in organic chemistry allow alkenes or alkynes to “swap” substituents. This can be useful, e.g., for the synthesis of unsaturated organic compounds or for polymerizations. Generally, transition-metal complexes based on metals such as ruthenium are used to catalyze olefin metathesis. Using Earth-abundant metals such as iron could lower costs and improve the sustainability. There has been some work on this topic, but the full potential of iron-based catalysts for olefin metathesis has not been explored so far.
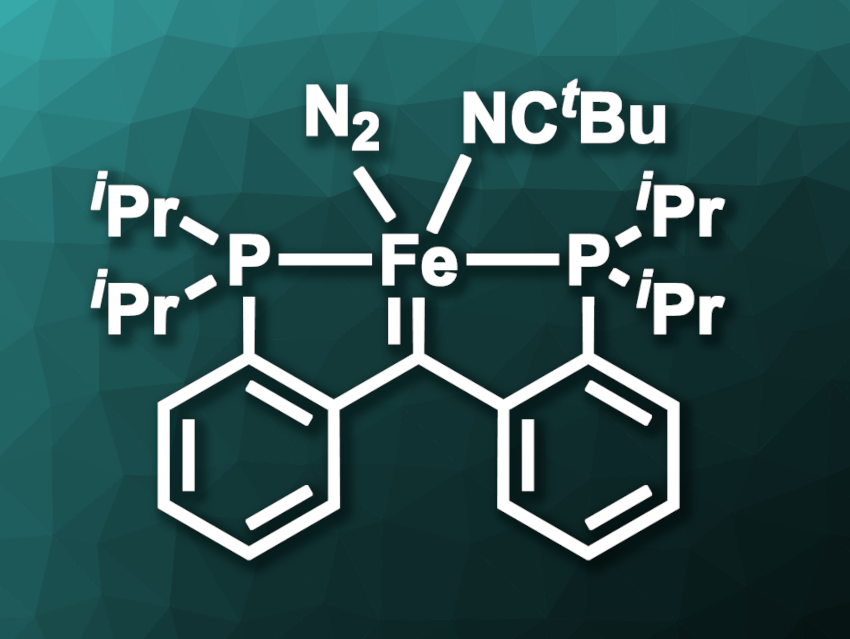
Vlad M. Iluc, University of Notre Dame, Indiana, USA, and colleagues have investigated the reactivity of iron carbene complexes of the type [{PC(sp2)P}Fe(L)(N2)], ([PC(sp2)P] = bis[2-(diisopropylphosphino)phenyl]methylene, L = PMe3, tBuCN, example pictured) and their potential for use in olefin metathesis. The team used norbornadiene (NBDE) derivatives as strained model alkenes.
The researchers found that the iron carbene complex with L[nbdp]=[nbdp]PMe3 did not react with the NBDE derivatives. They then tried using pivalonitrile (tBuCN) as a more labile ligand and found that the resulting iron carbene complex could react with NBDE and its derivatives. The team was able to isolate metallacyclobutane complexes that were formed in these reactions. They added 2,6-dimethylbenzonitrile as a ligand to the metallacyclobutanes formed in a reaction with 2,3-diphenylnorbornadiene (DPNBDE), and the reduced solubility of the resulting complex allowed them to obtain a solid-state structure.
In addition to the metallacyclobutanes, which are usually intermediates in the Chauvin olefin metathesis mechanism, the team also found evidence of a ring-opened product. Together, these results indicate that iron complexes can take part in olefin metathesis and that they can adhere to the Chauvin mechanism.
- Iron Olefin Metathesis: Unlocking Reactivity and Mechanistic Insights,
Zachary S. Lincoln, Vlad M. Iluc,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c04356