Metal-catalyzed nitrene transfer reactions can be useful for introducing nitrogen-containing groups directly into different molecules. Research on this topic has often focused on C−N bond-forming reactions such as C−H aminations. However, nitrene transfer reactions could also enable the formation of bonds between existing heteroatoms and nitrogen atoms, which is less well-explored. Iron-based catalysts are interesting in this context, and new types of ligands could expand the utility of this type of catalyst for nitrene transfers.
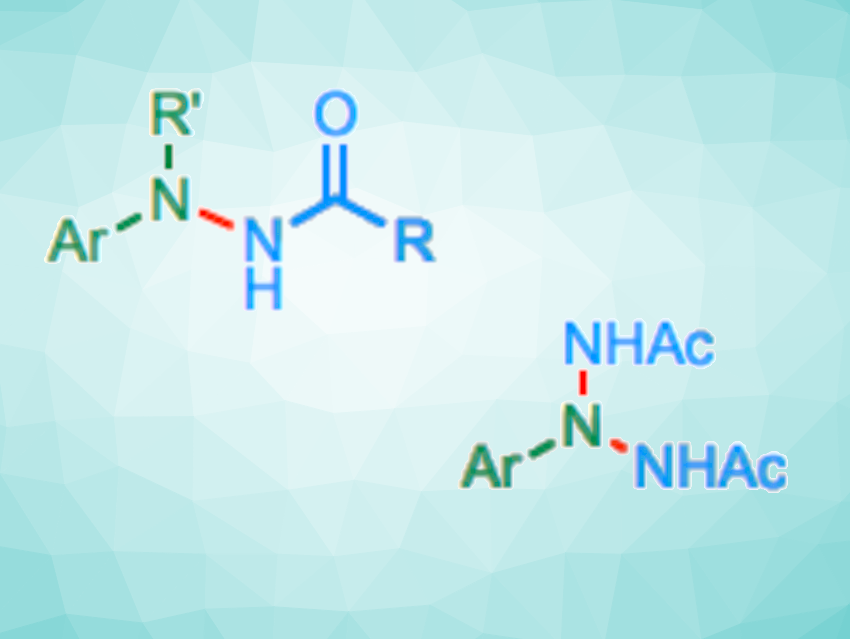
Hao Wang, Nankai University, Tianjin, China, Gong Chen, Nankai University and Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, China, and colleagues have developed an approach to the iron-catalyzed N-amidation of arylamines with 3-alkyl dioxazolones, giving hydrazides. In addition, triazanes can be accessed via a double or sequential N-amidation of primary arylamines (example reactions pictured below). The team found that bulky, strongly σ-donating alkylphosphine ligands (PtBu3) can promote this type of reaction, using FeCl2 as a catalyst precursor.
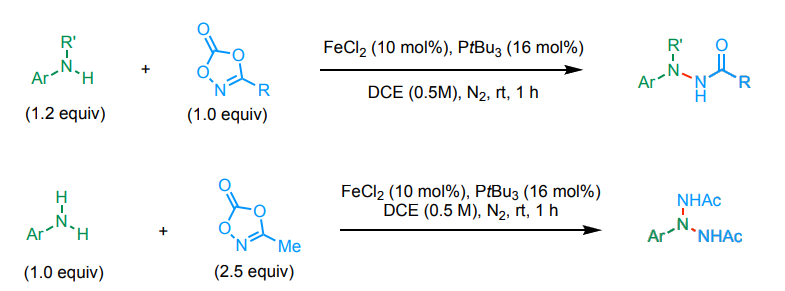
Based on mechanistic studies, the researchers propose that the phosphine ligands facilitate the decarboxylation of dioxazolones at the iron center. This leads to the formation of Fe nitrenoid intermediates, which then undergo N−N coupling reactions. According to the team, the work could be useful for further research focusing on saturated polynitrogen compounds.
- Ligand‐promoted Iron‐catalyzed Nitrene Transfer for the Synthesis of Hydrazines and Triazanes through N‐Amidation of Arylamines,
Shi-Yang Zhu, Wen-Ji He, Guan-Chi Shen, Zi-Qian Bai, Fang-Fang Song, Gang He, Hao Wang, Gong Chen,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202312465