Metal complexes have immense potential in cancer therapy, as demonstrated by the clinical success of platinum-based anticancer drugs and the development of metalloimmunology. Metalloimmunology is the use of metal compounds for the regulation of immunity, which could affect aspects such as the structures and functions of immune-related proteins or cellular responses. Microtubules are polymers of tubulin proteins. They are important, e.g., for maintaining cell shape and are involved in mitosis (cell division). Drugs that interact with these proteins can be useful in cancer treatment by disturbing cell division.
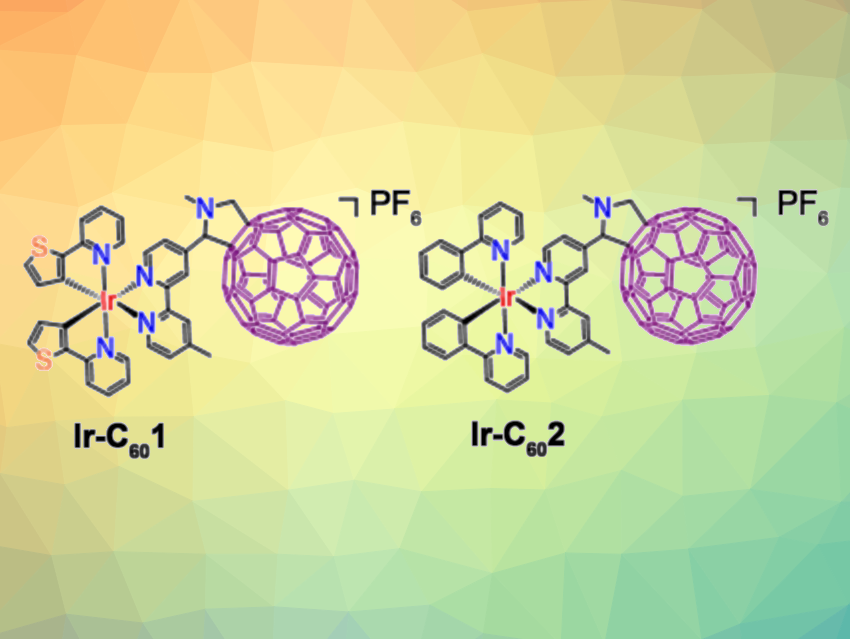
Jian-Zhang Zhao, Dalian University of Technology, China, Cai-Ping Tan, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed two fullerene (C60)-functionalized Ir(III) complexes (Ir-C601 and Ir-C602, pictured) that can serve as dual reactive oxygen species (ROS) regulators and microtubule-targeted photosensitizers. In the dark, the complexes serve as ROS scavengers to eliminate O2•− and •OH, which reduces cytotoxicity in the dark and can decrease the number of dysfunctional T cells (a type of immune cell). Under green light irradiation (525 nm), the complexes can be excited to produce O2•−, •OONO−, and 1O2 to kill cancer cells.
In addition, Ir-C601 can photooxidize tubulin and promote microtubule polymerization, interfering with the cellular structures, inducing immunogenic cell death, and inhibiting cell proliferation. Proteomic and RNA-sequencing studies confirm that Ir-C601-mediated photodynamic therapy can trigger cell death and affect immune-related pathways by inducing microtubule polymerization. The team used transferrins (a type of glycoprotein) to encapsulate and deliver Ir-C601 and found that this approach can significantly improve the tumor immune microenvironments in vivo in a mouse model. Overall, the work provides insights into the rational design of new targeted photoimmunotherapeutic agents.
- Microtubule polymerization induced by iridium‐fullerene photosensitizers for cancer immunotherapy via dual‐reactive oxygen species regulation strategy,
Xiao‐Xiao Chen, Kun Peng, Xi Chen, Zheng‐Yin Pan, Qing‐Hua Shen, Yu‐Yi Ling, Jian‐Zhang Zhao, Cai‐Ping Tan,
Aggregate 2024.
https://doi.org/10.1002/agt2.623