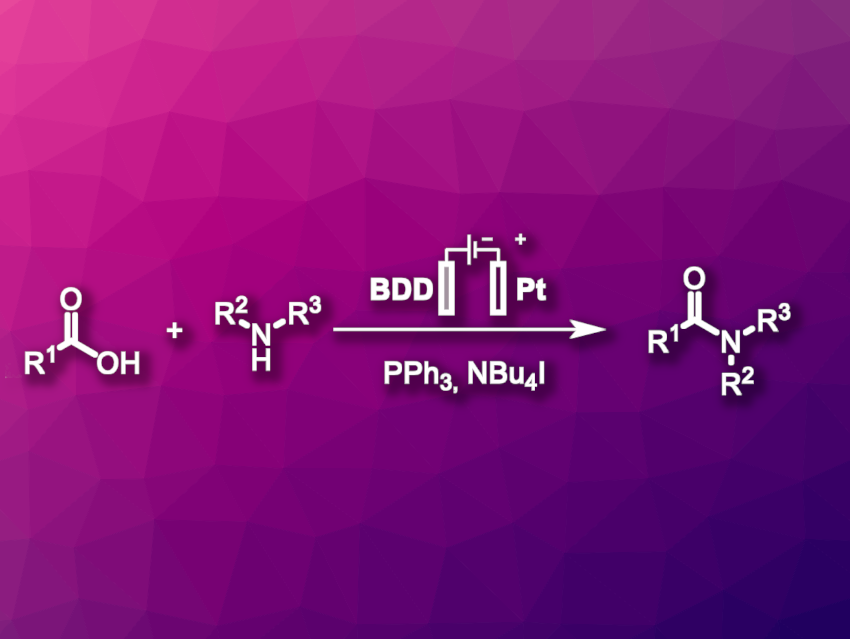
Amide bonds are very common functional groups. They are not only found in peptides but also, e.g., in pharmaceuticals or natural products. Conventional methods for the formation of amides generally rely on pre-activation of the acid component using hazardous coupling reagents, which often end up in hardly recyclable waste.
Till Opatz, University of Mainz, Germany, and colleagues have developed an electrochemical iodine-mediated amide coupling using triphenylphosphine as a coupling reagent (pictured). The team used a boron-doped diamond (BDD) anode to generate a reactive intermediate phosphorus species by anodic oxidation. This anode was combined with a Pt cathode and MeCN was used as the solvent. The anodically oxidized PPh3 activates the carboxylic acid for the desired reaction, and triphenylphosphine oxide is formed as a byproduct.
Iodide in the form of NBu4I was added to the electrolyte and used as a redox mediator. It lowered the required electrode potential for the oxidation. The team proposes a mechanism in which the iodide is oxidized to form triiodide. The triiodide then reacts with PPh3 to form an intermediate that reacts with the carboxylic acid and activates it.
The team used the protocol for reactions of a broad range of aromatic and aliphatic acids with primary and secondary amines and even amino acids to give the respective amides. The reaction can be performed on a gram scale. The electrochemical approach allowed the researchers to avoid the use of hazardous reagents for activation, while the use of iodide as a mediator allowed for the use of sensitive substrates. The byproduct triphenylphosphine oxide can be reduced and recycled, and the reaction can be controlled by the amount of current passed through the solution. The method could provide sustainable access to amides and might be useful for industrial applications.
- An Iodide‐mediated Anodic Amide Coupling,
Luca Marius Großmann, Vera Beier, Lea Duttenhofer, Laura Lennartz, Till Opatz,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201768