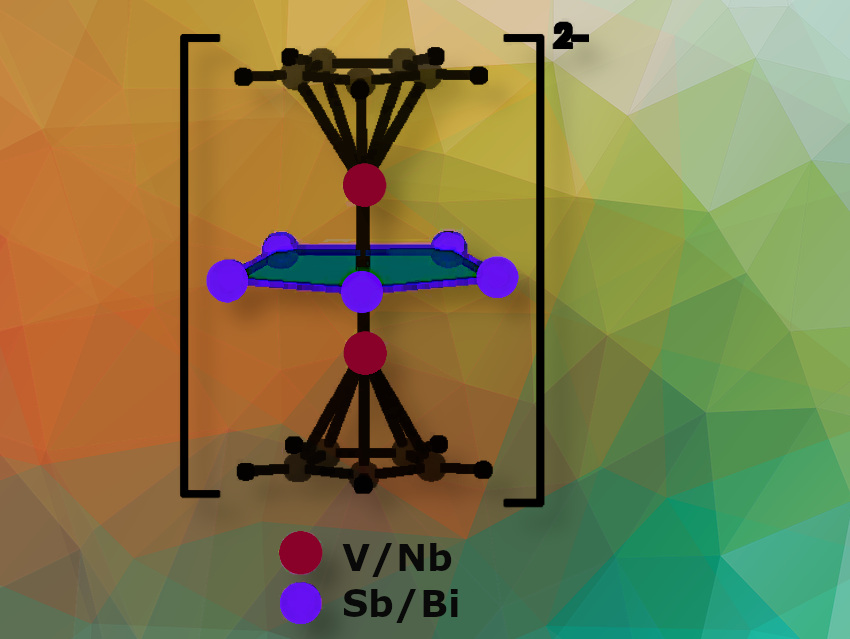
The cyclopentadienyl anion is a π-aromatic five-membered ring commonly used in organometallic chemistry. Inorganic versions have been created by replacing the CH groups with isoelectronic elements from group 15, such as phosphorus and antimony. These Pn₅ rings have been stabilized in a triple-decker sandwich structure, prepared at high temperatures. A bismuth (Bi₅⁻) ring has also been successfully stabilized in a cobalt-based inverse-sandwich-type complex.
Gernot Frenking, Nanjing Tech University, China, Philipps-Universität Marburg, Germany, and Donostia International Physics Center (DIPC), Spain, Zhong-Ming Sun, Nankai University, Tianjin, China, and colleagues have synthesized two complexes containing planar Sb₅ and Bi₅ rings under mild reaction conditions in a one-pot approach (pictured above). [Cp–V(cyclo-Sb₅)V–Cp]²⁻ and [Cp–Nb(cyclo-Bi₅)Nb–Cp]²⁻ are stabilized by [K(18-crown-6)]⁺ or [K(2.2.2-crypt)]⁺ cations.
The team used Zintl-phase compounds as precursors. The compound [K(18-crown-6)]₂.₅[Cp–V(cyclo-Sb₅)V–Cp]·0.5Cp·3.5Py was synthesized by reacting K₅SnSb₃ and VCp₂ in the presence of 18-crown-6. Similarly, reacting the Zintl-phase K₂SnBi with the organometallic precursor NbCp₄ in a mixture of en and [2.2.2]crypt solutions led to the formation of the complex [K(2.2.2-crypt)]₂[Cp–Nb(cyclo-Bi₅)Nb–Cp]·0.5en·1.5tol.
The core structure of the cluster is a pentagonal bipyramidal structure. Transition-metal atoms (V or Nb) cap both ends of the Pn₅ (Pn = Sb, Bi) ring. Unlike traditional cyclopentadienyl anions, these inorganic analogues lack aromaticity due to V–V and Nb–Nb bonds passing through the center of the pentagonal rings.
This study provides an alternative synthetic strategy and introduces new bonding models for planar clusters of antimony and bismuth, underscoring their unique coordination chemistry.
- Synthesis of triple-decker sandwich compounds featuring a M–M bond through cyclo-Bi5 and cyclo-Sb5 rings,
Yu-He Xu, Xing Yang, Ya-Nan Yang, Lili Zhao, Gernot Frenking, Zhong-Ming Sun,
Nature Chemistry 2025.
https://doi.org/10.1038/s41557-025-01765-4