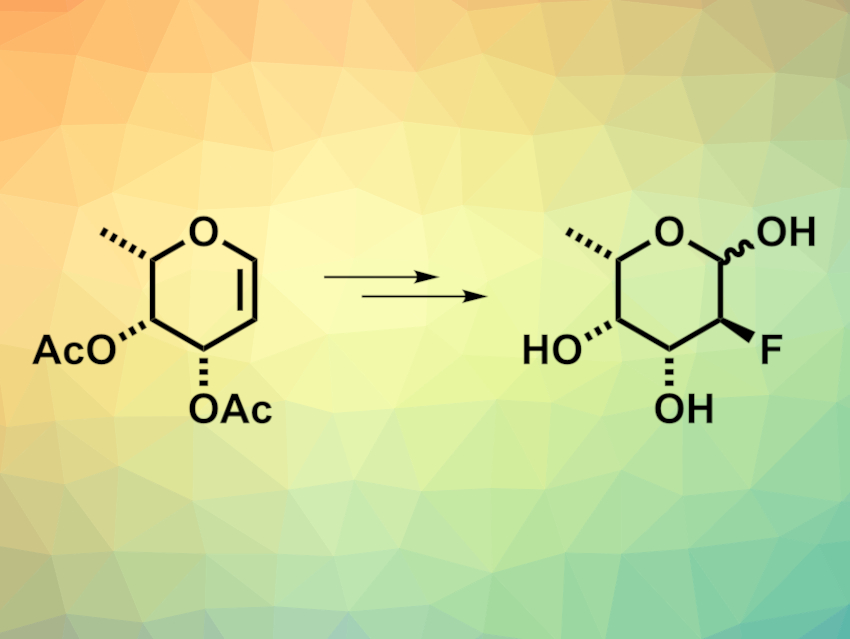
2-Fluorofucose, or SGD-2083 (pictured above on the right), can inhibit protein fucosylation. This, in turn, can affect tumor cells. Thus, 2-fluorofucose shows antitumor activity. However, the compound would require high doses for phase 1 trials, which makes it necessary to develop a robust process for the synthesis of large amounts of 2-fluorofucose. An existing process uses commercially available L-(−)-fucose as a starting material. However, the use of this substrate for large-scale synthesis is hampered by limited availability and high costs.
Bradley J. Paul-Gorsline, Seagen, Bothell, WA, USA, and colleagues have used another sugar as a viable starting material for preparing large amounts of 2-fluorofucose: L-(−)-rhamnose. According to the researchers, L-(−) rhamnose costs about USD 70/kg, compared with USD 1000/kg for L-(−)-fucose. They first methoxylated L-(−)-rhamnose, followed by the protection of two hydroxy groups as an acetal and a stereochemical inversion at the C4 position. The inversion was achieved by oxidation of the respective alcohol to a ketone, followed by a stereoselective reduction. A known glycal intermediate (pictured above on the left) was then obtained via an acetylation/bromination/elimination sequence.
The resulting glycal (SGD-2080) can be used as a key intermediate in the established synthesis of 2-fluorofucose, providing a scalable route to this possible anticancer agent with a reduced cost. The team obtained the desired precursor SGD-2080 in eight steps and an overall yield of 32 %.
- An Alternative Route to the Anticancer Agent 2-Fluorofucose from Readily Available L-(−)Rhamnose and Mechanistic Insights into a Zinc/Ammonium Iodide-Mediated Elimination Reaction,
Roozbeh Yousefi, Bradley J. Paul-Gorsline, Omid Soltani, Kumar D. Ashtekar,
Org. Proc. Res. Dev. 2022.
https://doi.org/10.1021/acs.oprd.2c00146