Proton-coupled electron transfer (PCET) is a light-induced process that is important in chemistry and biology. PCET processes are often found in biochemical transformations, but can also be used to convert light energy into chemical energy, which is contained in photoproducts.
Olaf Morawski, Institute of Physics, Polish Academy of Sciences, Warsaw, and colleagues have investigated the photochemical properties of an electron-deficient organic chromophore with six nitrogen atoms—hexaazatrinaphthylene (HATN)—in different alcohols and in water. HATN was obtained by a condensation reaction between hexaketocyclohexane octahydrate and an excess of 1,2-phenylenediamine. The properties of this molecule were studied using optical, NMR, and EPR spectroscopy, as well as mass spectrometry techniques.
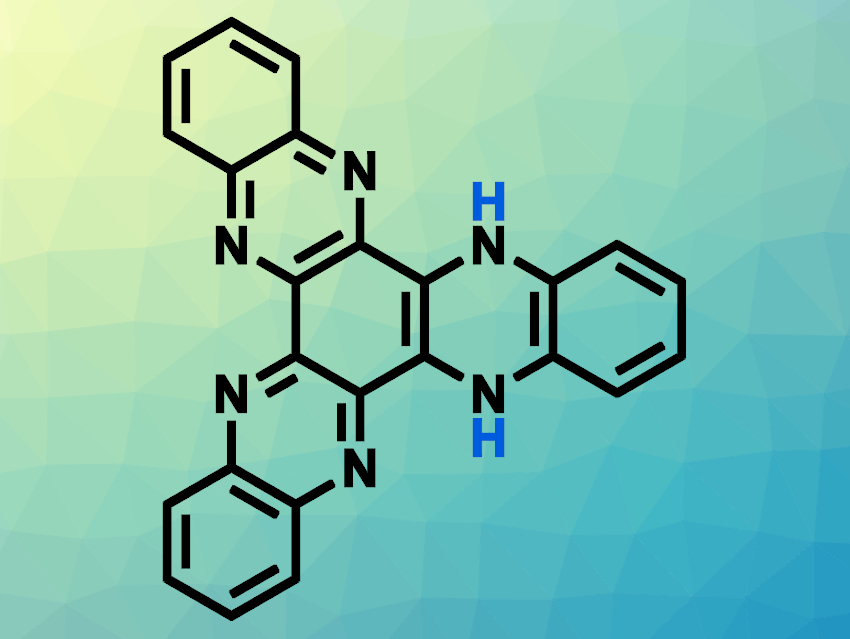
The team found that HATN, when irradiated with violet light, abstracts a hydrogen atom from methanol, forming a biradical pair: hydrogenated HATN (HATN–H•) and a methoxy radical (MeO•). This photochemical hydrogenation occurs in several alcohols (methanol, ethanol, isopropanol) but not in water. This intermolecular PCET between HATN and the solvent is the first step of the photochemical reactions. Subsequently, dihydrogenated HATN (HATN–2H, pictured above) was formed, possibly via a disproportionation of the monohydrogenated product. HATN–2H is a stable structure that could be useful for long-term hydrogen storage.
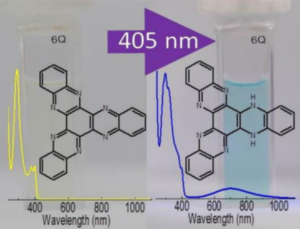
- Photochemical Hydrogen Storage with Hexaazatrinaphthylene (HATN),
Olaf Morawski, Paweł Gawryś, Jarosław Sadło, Andrzej L. Sobolewski,
ChemPhysChem 2022.
https://doi.org/10.1002/cphc.202200077



The first radical is almost certainly *CH2OH (where * = single electron)