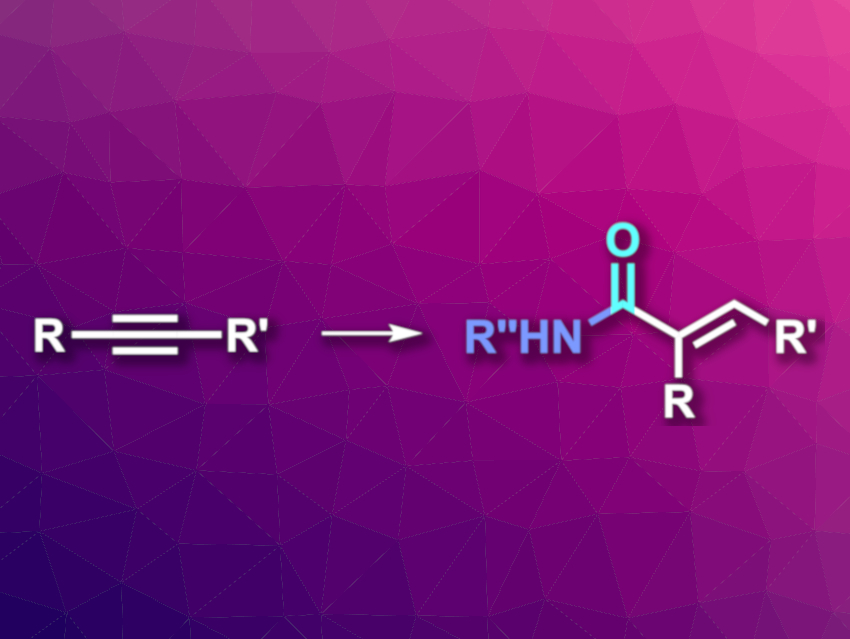
Hydroaminocarbonylation reactions can be used to transform alkenes or alkynes, carbon monoxide, and amines into amides in a step- and atom-economical manner. When alkynes are used, the products are α,β-unsaturated amides, which can be useful for further transformations. Regio- and stereoselective variants of this type of reaction are generally limited to terminal alkynes so far.
Xiao-Feng Wu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Leibniz-Institut für Katalyse e. V., Rostock, Germany, and colleagues have developed a regio- and stereoselective hydroaminocarbonylation of unsymmetrical internal alkynes (pictured). The team used Pd(PPh3)4 as the catalyst together with xantphos as a bidentate diphosphine ligand and p-toluenesulfonic acid monohydrate (PTSA). The reactions were performed in tetrahydrofuran (THF) under 10 bar CO at 100 °C.
The team used the approach to react a variety of substituted anilines, aromatic secondary amines, heteroarylamines, and hydrazines with different unsymmetrical alkynes. The desired α,β-unsaturated amides were obtained in moderate to excellent yields, with excellent regioselectivity, and exclusive (E)-stereoselectivity. The reaction has a broad substrate scope and good functional group tolerance.
- Palladium-Catalyzed Regio- and Stereoselective Hydroaminocarbonylation of Unsymmetrical Internal Alkynes toward α,β-Unsaturated Amides,
Chang-Sheng Kuai, Jian-Xing Xu, Bo Chen, Xiao-Feng Wu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01693