Atomically precise copper nanoclusters can be more challenging to synthesize than nanoclusters made from noble metals. Copper nanoclusters can be useful, e.g., in catalysis. Often, they are prepared using reducing agents that contain hydrides, which leads to the incorporation of hydrides into the cluster structure. This can affect their catalytic function. New methods for the synthesis of hydride-free copper nanoparticles could, thus, be useful.
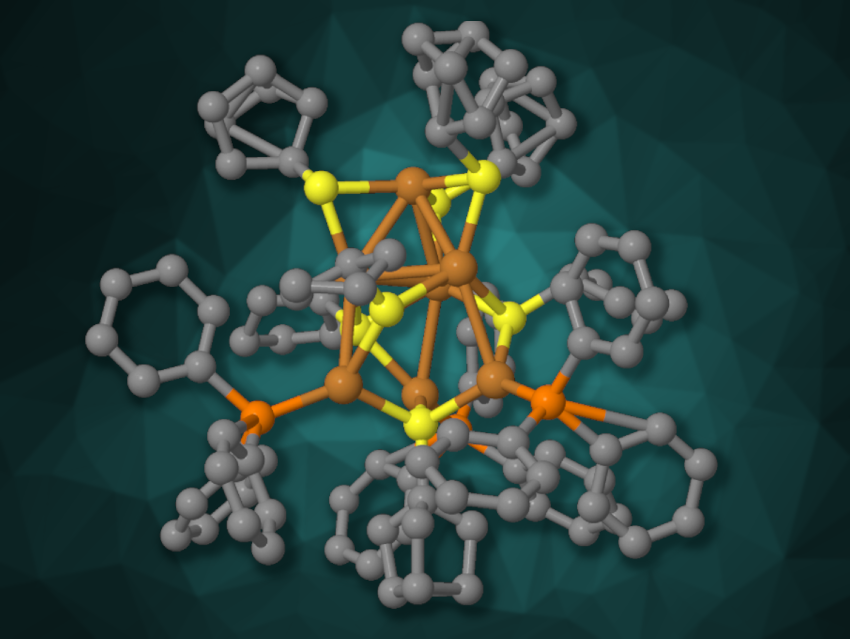
Basudev Sahoo, Indian Institute of Science Education and Research Thiruvananthapuram, Kerala, India, Biswarup Pathak, Indian Institute of Technology Indore, Madhya Pradesh, India, Yuichi Negishi, Tokyo University of Science, Japan, and colleagues have synthesized a hydride-free copper nanocluster, [Cu7(SC5H9)7(PPh3)3] (simplified structure pictured), and used it to promote C–C cross-coupling reactions between terminal alkynes and aryl halides. The team used Cu(CH3CN)4BF4 as a copper precursor, which was reacted with triphenylphosphine and cyclopentanethiol, followed by the addition of sodium borohydride and recrystallization.
The product was characterized using single-crystal X-ray diffraction, and the researchers found that four of the cluster’s seven copper atoms are arranged in a triangular pyramid, with the three remaining copper atoms connected to its “base”. The cluster showed photoluminescent properties. It was used for photoinduced C–C coupling reactions under purple LED light. The team reacted different aryl halides with terminal (hetero)aromatic alkynes and obtained the desired coupling products in mostly high yields.
- Luminescent Hydride-Free [Cu7(SC5H9)7(PPh3)3] Nanocluster: Facilitating Highly Selective C–C Bond Formation,
Sourav Biswas, Amit Pal, Milan Kumar Jena, Sakiat Hossain, Jin Sakai, Saikat Das, Basudev Sahoo, Biswarup Pathak, Yuichi Negishi,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c05678