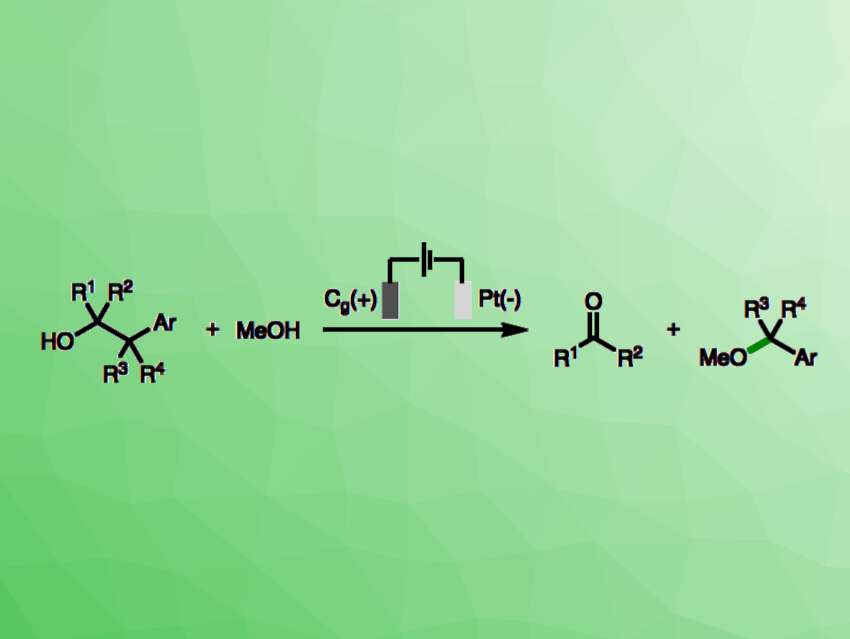
Electrochemistry can be useful in organic synthesis by generating radical intermediates that can then take part in a vareity of reactions. This can be used, for example, for the deconstructive methoxylation of arylalcohols (general reaction pictured). However, this type of reaction comes with unanswered mechanistic questions, such as which part of the molecule undergoes anodic oxidation or whether the following fragmentation of the carbon skeleton and nucleophilic attack occur in the same step.
Matthew N. Grayson, University of Bath, UK, Louis C. Morrill, Cardiff University, UK, and University of Bath, and colleagues have used synthetic, electroanalytical, and computational experiments to investigate this reaction mechanism. The team initially considered three mechanisms for the C–C bond cleavage of the employed 2-arylalcohols: 1) an anodic oxidation of the hydroxyl group followed by a mesolytic C–C bond cleavage (i.e., a bond cleavage that gives a neutral radical and an ion), 2) an anodic oxidation of the aromatic ring followed by a mesolytic C–C bond cleavage (an SN1-like pathway), or 3) an anodic oxidation of the aromatic ring followed by an intermolecular nucleophilic attack (an SN2-like pathway).
The team found that the reaction likely starts with an anodic oxidation of the aromatic ring to form the corresponding aromatic radical cation based on the results of cyclic voltammetry experiments, among other methods. Density functional theory (DFT) calculations were used to evaluate the stability of possible radical and cationic fragments that would be formed by mesolytic cleavage of the aromatic radical cations, indicating the involvement of oxocarbenium ions and benzylic radicals. In addition, synthetic experiments confirmed that the electrochemical fragmentation of the 2-arylalkanols requires structural features that stabilize oxocarbenium ions and/or benzylic radical intermediates. Further anodic oxidation and trapping of the resulting benzylic carbocation with methanol then gives the desired methyl ether products.
According to the researcher, the work provides an improved understanding of the reaction mechanism and the structural features that promote fragmentation. This could be useful for the development of further electrosynthetic transformations based on this mode of substrate activation.
- Electrochemical Deconstructive Methoxylation of Arylalcohols – A Synthetic and Mechanistic Investigation,
Hussain Maashi, Toby Lewis-Atwell, James Harnedy, Matthew Grayson, Louis Christian Morrill,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403413



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)