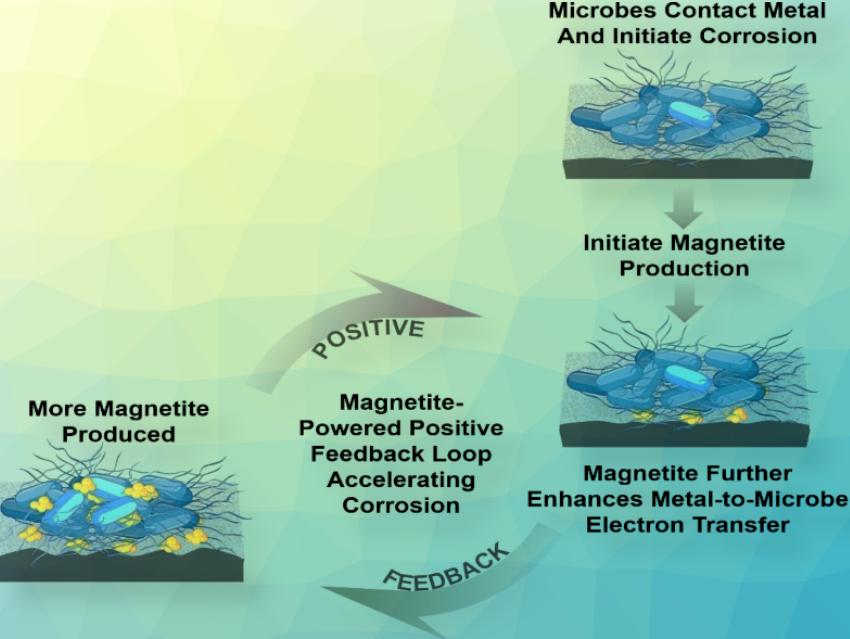
Iron is well-known for rusting, but this doesn’t just happen on contact with oxygen and water. Some bacteria are also able to decompose iron anaerobically in a process referred to as electrobiocorrosion. The sediment-dwelling bacterium Geobacter sulfurreducens uses electrically conductive protein threads for this purpose. They produce magnetite from the iron, which promotes further corrosion in a positive feedback loop.
Electrobiocorrosion
Bacterial biofilms are the cause of microbial metal corrosion, a destroyer of metals which causes more costly damage than all other biofilm-related damage put together. Electrobiocorrosion is often caused by bacteria such as those found in river sediments, for example, the anaerobic genus Geobacter. Geobacter does not use atmospheric oxygen for respiration; instead, it draws energy from the transfer of electrons from iron (Fe0), forming magnetite in the process. Thus far, the way in which Geobacter corrodes iron metal has been something of a mystery.
The exact mechanism of action of electrobiocorrosion has now been investigated more closely by Dake Xu and colleagues from Northeastern University in Shenyang, China. The team worked on the assumption that electrically conductive pili (e-pili), thin filaments which grow out of the bacteria, could play an important role in this mechanism.
The Role of Electrically Conductive Pilli
Geobacter forms “e-pili” from conductive proteins. These e-pili act like electric wires, conducting electricity. Before this study, it was unclear whether the e-pili could withdraw electrons directly from metal surfaces.
To prove the team’s suspicions, namely direct electron withdrawal, they left two strains of Geobacter to grow on a stainless-steel surface until biofilms formed. One of the two strains formed conductive e-pili, while the other still produced pili, but had been genetically modified so that the pili were formed from less conductive proteins. The researchers observed that the bacterial strain that grew e-pili fared significantly better on the steel plate. It grew more and made deeper pits in the metal, demonstrating how much metal it was consuming. The team also measured a corrosion current, a direct sign of the oxidation of iron.
The team concluded that the bacteria with the e-pili formed a sort of “electrical connection” to the metal. Bacteria located further away in the biofilm, not in direct contact with the metal, were also able to supply themselves with electrons using e-pili.
Magnetite
Because magnetite is formed during the corrosion of iron, and this mineral also conducts electricity, the team also investigated its influence on microbial corrosion. They noted that not only did adding magnetite to the biofilm increase the growth of Geobacter, it also led to a stronger corrosion current measured at the surface of the metal. Magnetite reduced resistance to electron transfer, increasing corrosion currents and intensifying pitting. As corrosion proceeds, additional magnetite may be generated, strengthening a positive feedback loop to further accelerate corrosion.
In the presence of magnetite, the need for cells to produce biological electrical contacts may be diminished, reducing the cell’s biosynthetic energy requirements, potentially leading to faster cell growth and further hastening corrosion. The extent of magnetite formation during iron corrosion is expected to be highly dependent upon conditions near the corroding metal surface. Therefore, material modifications or other strategies to prevent magnetite formation might be successful in arresting electrobiocorrosion.
Accelerated Microbial Corrosion by Magnetite and Electrically Conductive Pili through Direct Fe0-to-Microbe Electron Transfer,
Yuting Jin, Enze Zhou, Toshiyuki Ueki, Danni Zhang, Yongqiang Fan, Dake Xu, Fuhui Wang, Derek R. Lovley,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202309005